Introduction
Clinician’s decision mostly depends on laboratory result for screening, diagnosing & monitoring of diseases. Thus it is important for the clinical laboratories to produce reliable, reproducible, accurate test results so that both patients and clinicians can rely upon reports.
But all laboratory procedures are prone to errors because of higher rate of human intervention in each step of analysis even in the presence of automated analysers. So the laboratories must establish and maintain quality in all laboratory process to ensure the quality of report. For this quality control validation has been used by all laboratories for clinical interpretation of the test. Quality control is performed for two purposes – to detect errors and to avoid false rejections. So the laboratories use internal quality control and external quality assessment system(EQAS) to calculate various scores like Bias and CV%(coefficient of variation) to evaluate quality of their reports.
Internal quality control which is performed daily, helps in deciding whether the results are reliable enough to be released to the physician. On the other hand EQAS is done by the third party on a monthly basis and gives information on the accuracy or bias in the system & methods used in respective lab. Both are expensive exercises and carry considerable cost to laboratory especially if repeat analysis are done in each category. It is therefore necessary to identify tests that require more quality control runs per day. One of the methods in prioritizing which tests are at risk is known as six sigma and it is part of the lean laboratory practice which focuses on cost effectiveness in quality improvement.
Six sigma system is an evolution in quality assessment & management that has been implemented widely in business and industries in the mid-1980s. It was developed by Motorola to reduce cost of products, eliminate defects, decrease variability in processing and it provides a more quantitative frame work for process performance.1 Six sigma is usually applied to tests with high volume and high impact on patient care. The Six Sigma model has an extra step, control, when compared to total quality management (plan, do, check, and act)which is important in modern quality management. With this step, we intend to prevent defects from returning to the process. There are a few studies done on sigma metrics in laboratory medicine.2, 3, 4
Sigma is a uniquely defined scale with which we can assess the performance of a lab. It evaluates the process by counting defects and converting it into defects per million opportunities rate as follows.5
Table 1
Shows the errors per million reports corresponding to each sigma value.5
| Sigma (σ) | Errors/million reports |
| 1 sigma | 690000 |
| 2 sigma | 308000 |
| 3 sigma | 66800 |
| 4 sigma | 6210 |
| 5 sigma | 230 |
| 6 sigma | 3.4 |
Scaling of Sigma indicates how often errors are likely to occur : the higher the sigma value, chance of false test results is less likely. When performance falls below 3 sigma, the process is considered as unstable and unacceptable and should not be used for routine test purposes.6, 7, 8 Therefore an analytical procedure should achieves a good sigma levels for a quality (reliable & accurate) report & it is the responsibility of laboratories to keep professional standards and maintain the quality of procedures.
So as a part of quality improvement in the laboratory, this study has been done to calculate sigma metrics for RFT(Renal function test) & Electrolyte analytes in our laboratory and to use it as a self-assessment tool to analyse quality control strategy followed in the clinical chemistry laboratory to plan QC(quality control) frequency accordingly.
Materials and Methods
This observational Study has been conducted in clinical Biochemistry laboratory, K R hospital Mysore. Internal quality control (IQC) data (Bio-Rad) for RFT & Electrolyte (5) parameters analysed retrospectively over a period of 6 months (181 days - level 1 and level 2 values) in fully automated modular equipment (COBAS-6000). Institutional ethical committee clearance had been obtained to carry out the study.
Inclusion criteria
The analytes included were internal quality control data (Level 1, Level 2) of Urea, Creatinine, Sodium, Potassium, Chloride from January 2019 to June 2019 (181 days).
IQC used to calculate bias, CV%, mean & standard deviation for each levels. Total allowable error (TEa) values of various parameters were taken from Clinical Laboratories Improvement Act (CLIA) guidelines.9
Sigma value is calculated by the equation Sigma = (TEa- bias) / CV%
Statistical analysis is done in excel sheet. Sigma values were represented in graphs from January 2019 to June 2019 for each analytes. Operating point for each analytes were plotted in graphs with the help of excel sheet from Westgard for quick view.10
Results
Table 2
TEa%, Bias%, CV% for different parameters for Level I (L1)&Level 2(L2) in COBAS 6000 (January 2019 to June 2019)
Table 2 shows the TEa obtained from CLIA guidelines, Bias and CV value of different parameters. Out of 5 parameters analysed, highest BIAS & CV was observed in creatinine values in both Level 1 & Level 2 QC.
Table 3
Sigma value for different parameters for Level I & Level 2 QC in COBAS 6000 (January 2019 to June 2019)
Figure 1
Sigma value for different parameters for Level I (SIGMA 1)& Level 2(SIGMA 2) QC in COBAS 6000 (January 2019 to June 2019)
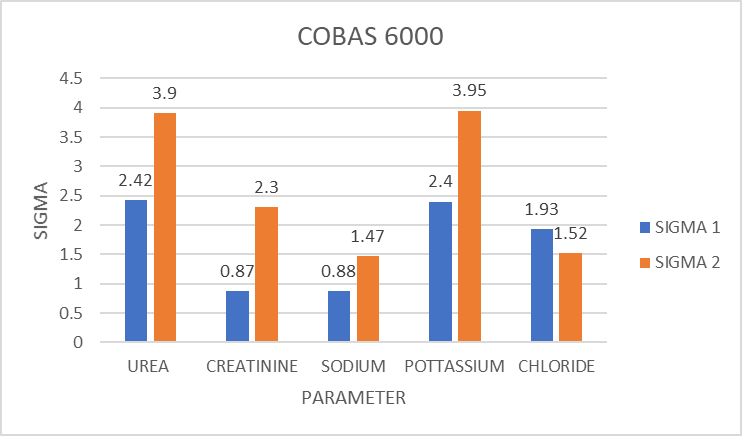
Table 3 & Figure 1 depicts the sigma value obtained for different parameters in Level 1 & Level 2 QC in COBAS 6000. It shows that no parameter has got sigma value above 6. Parameters which showed sigma value between 3-6 are Urea & Potassium in Level 2 QC. Those Parameters which showed sigma value below 3 are Urea, Creatinine, Sodium, Potassium & Chloride in Level 1, creatinine, sodium & chloride in Level 2 QC.
Figure 2 shows Operating point for urea and pottasium plotted by using Six Sigma Medex chart from Westgard. X axis shows allowable imprecision and Y axis shows allowable inaccuracy or bias. This give a quick view on different parameters regarding where the operating point lies with respect to sigma value.10
Discussion
Six sigma has paved a new path in the world of quality as it sets a quality baseline. Each and every laboratory can design their own quality control strategy by using sigma value which helps in evaluating the laboratory performance. Six sigma helps in evaluating the laboratory performance based on the sigma values and with the help of westgard operational specifications chart (OPSpecs chart), Schoenmaker et al. specified importance of sigma metrics application and its use in designing QC. Six sigma aims at monitoring a process to 6 SDs, representing 3.4 DPM(defects per million) opportunities.11, 12
After obtaining a sigma value for a particular parameter we can select the Westgard rule with which the assessment of the same parameter can be done. With the Sigma Standard Quality Control selection tool, a power function graph one can assess the maximum error detection which will help us to improve the quality of reports.13
This study analysed 5 analytes over a period of 6 months (January – June 2019) and assessed for sigma metrics. Similar studies were done by Usha S et al., Vijatha et al., Nikunj et al., Justice Afrifa et al. Bhavna Sing et al., Sunil Nanda et al. etc.1, 2, 3, 4, 5, 14, 15 Variations in sigma values between this study and others can be attributed to the difference in the instrument used, quality control material used and other pre & post analytical conditions.
In this study highest bias value was for creatinine in both the instruments. So there is chances of inaccuracy in the methods for measurement of creatinine which needs evaluation.
The Six Sigma scale typically runs from zero to six. In industries outside healthcare, 3 Sigma is considered the minimal acceptable performance for a process. When performance falls below 3 Sigma, the process is considered unstable and unacceptable and should not be used for routine test purposes. In contrast to other industries, healthcare and clinical laboratories appear to be operating in a 2 to 3 Sigma environment. Parameters whose sigma is > 6, stringent internal QC rules need not be adopted. In such cases, false rejections can be minimized by relaxing control limits up to 3SD.
Parameters which showed sigma value below 3 in L1 QC – Urea, Creatinine, Sodium, Potassium & Chloride, & in L2 QC– creatinine, sodium & chloride. We have also observed difference in sigma value for the same parameter in different QC levels.(fig 2). This is where the significance of Sigma matters in assessing quality control practices. The reason for the difference could be preparation of QC, batch no, or the methodology. This should be considered and root cause analysis has to be done. It is of utmost important to practice stringent maintenance of ISE unit to alleviate inaccuracies resulting in poor performance of ISE module
The parameters which demonstrated wide variation in the sigma values for both the levels of QC should be evaluated with discretion. The methodology should be re-evaluated. There is also a need to strictly follow Westgard multi rules as well as increase the number of QC runs so as to abolish this discrepancy. Similar to this study Nikunj Modi et al., also obtained different sigma value for same parameter in different levels.
So those parameters which have got sigma value <3 have to be reported carefully and identify the root cause & take the corrective action in order to improve the quality of reports in laboratory. These analytes require 8x along with 13s,R4s,22s,41s Westgard rules to apply on internal QC to achieve the improved sigma value. Also revision in the daily work load division is also recommended by Westgard sigma rules.13 We also need to make changes in our protocol of number of shifts in a day. For analytes with the outcome of sigma values less than 3, a change in the frequency of daily run is also required in our laboratory. The frequency would be required to change as 2 (12 hourly) or 4 (6 hourly) runs per day. In addition, 4 or 2 control measurements of each level. Therefore by using Standard QC selection tool and Westgard sigma rule we will be able to select the right QC (frequency of internal quality control & number of control) for instrument & laboratory. Thus sigma metrics in combination with a rational QC design for each analyte can improve the quality there by reducing the wastage.16
So ultimately our plan is to apply QC rules as mentioned by Westgard sigma rule.13
Sigma > 6 - apply 13s rule, 2 controls - no of runs – 1.
Sigma – 5 - apply 13s,R4s,22s, 2 controls - no of runs – 1.
Sigma – 4 - apply 13s,R4s,22s,41s, 2 controls - no of runs – 2 or 4 controls - no of runs – 1.
Sigma <3 - apply 13s,R4s,22s,41s,8x 4 controls - no of runs – 2 or 8 controls - no of runs – 1.
This is a pilot study. It requires a further analysis for a period of 6 months after applying the Westgard sigma rule as mentioned and after corrective action.
Conclusion
Six sigma is an easy way of streamlining the routine test procedures as it helps for assessing and comparing the performance of various tests using IQC, peer comparison and proficiency testing in the form of EQAS in the laboratory. With routine six sigma practice, the 2SD QC practices can be replaced with appropriate control limits and control measurements. It can be a more efficient way to assess the quality by matching the QC rules to the analytical quality of each individual assay. Each and every laboratory can use sigma metrics as guideline and quality baseline for quality control strategy. It can be used as a self-assessment tool regarding the performance of clinical laboratory & to check the reliability of report.


