Introduction
Breast cancer is a malignant proliferation of epithelial cells lining the ducts or lobules of the breast.1, 2 Human breast cancer is a colonial disease.3 A single transformed cell is the product of a series of somatic (acquired) or germ line mutations is eventually able to express full align ant potential. Thus, breast cancer may exist for a long period as either a non invasive disease or an invasive but non metastatic disease.4, 5, 6 These facts have significant clinical ramifications.7 Rising numbers of cases of breast cancer in India are the more young ladies affected. Lack of awareness and late presentation cause carcinoma.8, 9 Screening is the single most important factor responsible for better survival of patients in the carcinoma. Generally, the younger the age below menopause are the more aggressive of the cancer.10, 11, 12 A recent article of American Society of Clinical Oncology states that the overall 5-year survival rate of breast cancer patients in the United States, is from 75% to 89%. As a result of improved treatment and earlier detection, mortality from breast cancer has begun to decrease substantially.13 Cancer also known as malignant neoplasm, is a broad group of various diseases. In cancer, cells divide and grow uncontrollably, forming malignant tumours, and invade nearby parts of the body. There are over 200 different known cancers that afflict humans. Malignant neoplastic lesions are the eighth leading cause accounting for 6.7% of the total medically certified deaths in India.14, 15, 16, 17 Breast cancer is by far the most frequent cancer among women with an estimated 2.58 million new cancer cases diagnosed in 2019 (23% of all cancers), and ranks second overall (10.9% of all cancers).18, 19, 20 It is now the most common cancer both in developed and developing regions with around 690000 new cases estimated in each region (population ratio1:4).21 Breast cancer is the Most Common cancer in all urban areas in India, and 2nd most common in the rural areas.22 The most recent data Population Based Cancer Registry PBCR 2016 - 2018 tells us that breast cancer accounts for 25% to 31% of all cancers in women in cities (Mumbai, Delhi, Chennai, Ahmedabad, Bengaluru, Bhopal etc.). Breast cancer alone accounts for around 14% of total female neoplasm deaths.23, 24, 25
Materials and Methods
A total number of one hundred and fifty suspected Breast Carcinoma subjects were enrolled, out of these forty-five suspected cases were found positive and remaining one hundred and five subjects were taken as healthy controls from the Department of Gynaecology and Obstertrics, Osmania General Hospital (a teaching hospital to Osmania Medical College), Hyderabad, Telangana State, India. The various Departments are involved in this study for early prognosis and treatment.26, 27, 28
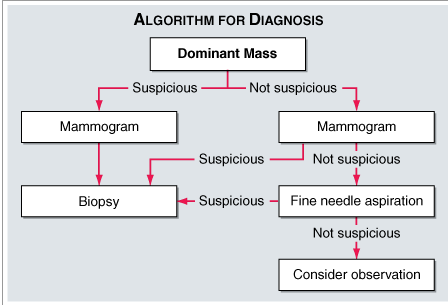
Tests and procedures used to diagnose breast cancer include:
Breast examination
A thorough physical examination was done to see any physical abnormalities are appeared and the suspected Breast Cancer are referred further for Mammography.29
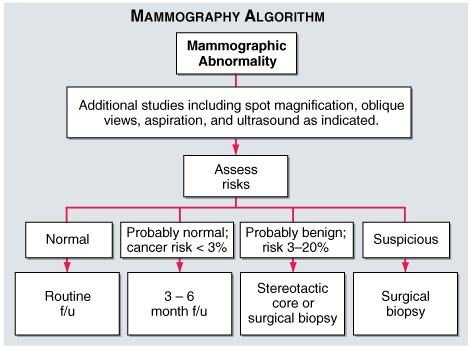
Mammography
A mammogram is an X-ray of the breast. Mammograms are commonly used to screen for breast cancer. If an abnormality is detected on a screening mammogram, a diagnostic mammogram is done to further evaluate that abnormality for Breast ultrasound. MRI also a latest tool for the early diagnosis of Breast Cancer. Biopsy is also sent for more confirmation in Diagnosis of Breast Cancer.30, 31, 32
Biochemical markers
Several tumour markers are in vogue now a days, which can be used for the detection of risk, population screening, diagnosis, staging and prognosis. They can also predict the response to therapy, monitor treatment, detect the presence of occult metastatic disease and monitor the course of the disease.33, 34
Though most of these investigations are highly specific and sensitive, the analytical method of many of these are unapproachable for general population as the facilities for these are available only at sophisticated and well-equipped centres with latest technology and are expensive. There is, therefore need of simple biochemical parameters, which are accurate, can be easily estimated, validated, and are less expensive.35
Serum Ferritin is elevated in Breast cancer and levels suggest the severity of the disease. Weinstein et al. found that malignant tissue had six times the Ferritin concentration. This has been attributed to the iron requirement for cell growth and malignant cells requiring more iron to modulate transferrin receptor.36
Malignancies with highest Ferritin concentration were more anaplastic suggesting major site for increased Ferritin is the malignant epithelium therefore it is postulated that Ferritin may be a marker of neoplasia. 37
Under normal conditions tissues maintain steady and consistent enzymatic pattern which is altered when tissue undergoes malignant change (seth ijms). Released cellular enzymes can be detected in plasma. Lactate dehydrogenase, Gamma glutamyl transpeptidase are two such enzymes. 38
Lactate dehydrogenase is an enzyme involved in anaerobic Glycolysis. Increase in Lactate Dehydrogenase levels is linked to poor prognosis.39
Gamma glutamyl transferase is an enzyme produced in several organs though mainly in liver and kidney. It is involved in amino acid transport through plasma membrane. Gamma glutamyl transferase is the best known marker for alcohol abuse however increased Gamma glutamyl transferase levels are seen in Breast Cancer with liver metastasis and can be included in the routine work up of patients of all stages of cancer. In view of this the present study was undertaken to evaluate the role of Ferritin, Lactate dehydrogenase and Gamma glutamyl transferase in breast cancer patients and to assess their value in early detection, monitor therapy and progression. 40
The data was analysed using Graph Pad Prism 6.0 Demo version software and the results were expressed as Mean and standard deviation of various parameters in different groups. Standard error of mean and coefficient of variation were also calculated. Unpaired t test was done to compare the cases and the controls, p value of ˂ 0.05 is considered as significant. Pearson’s correlation was done to assess the correlation of parameters within each group.ROC curve analysis was done to assess maximum sensitivity and maximum specificity and diagnostic efficiency of each parameter.
Table 1
Descriptive statistics of controls
Table 2
Descriptive statistics of cases
Table 3
Ferritin in control’s and case’s (Unpaired t-test between controls and cases)
|
|
Control’s |
Cases |
Welch corrected t, df |
P-value |
Significantly different (P < 0.05) |
|
Mean |
92.46 |
452.0 |
t=13.44 df=49.57 |
< 0.0001 |
Yes Two tailed P- value |
|
SD |
43.89 |
174.0 |
|||
|
SEM |
6.542 |
25.94 |
The mean value of Ferritin is elevated in cases than in the control group. This raise of Ferritin concentration in case group when compared to control group is statistically highly significant (p value < 0.0001). (Table 3)
Table 4
Lactate dehydrogenase in control’s and case’s
(Unpaired t-test between controls and cases)
|
|
Control’s |
Case’s |
Welch corrected t, df |
P-value |
Significantly different (P < 0.05) |
|
Mean |
296.7 |
613.9 |
t=12.44, df=46.51 |
< 0.0001 |
Yes Two tailed P- value |
|
SD |
28.48 |
168.6 |
|||
|
SEM |
4.245 |
25.14 |
The mean value of Lactate dehydrogenase is elevated in cases than in the control group. This raise of Lactate dehydrogenase in case group when compared to control group is statistically highly significant (P value < 0.0001).(Table 4)
Table 5
Gamma glutamyl transferase in control’s and case’s (Unpaired t-test between controls and cases)
|
|
Control’s |
Case’s |
Welch corrected t, df |
P-value |
Significantly different (P < 0.05) |
|
Mean |
14.39 |
60.64 |
t=18.54, df=49.45 |
< 0.0001 |
Yes Two tailed P- value |
|
SD |
4.04 |
16.24 |
|||
|
SEM |
0.6036 |
2.42 |
The mean value of Gamma glutamyltransferase is elevated in cases than in the control group. This raise of Gamma glutamyltransferase in case group when compared to control group is statistically highly significant (P value < 0.0001).(Table 5)
Table 6
Pearson’s correlation coefficient for analyzed parameters in control’s
Pearson’s correlation of controls
All the analyzed parameters have a positive correlation with each other.
Lactate dehydrogenase is statistically significantly positively correlated with Gamma glutamyltransferase.
Gamma glutamyltransferase is statistically significantly positively correlated to Lactate dehydrogenase.
Table 7
Pearson’s correlation coefficient for analyzed parameters in Case’s
Pearson’s correlation of case’s
Ferritin is statistically significantly positively correlated with Lactate dehydrogenase.
Ferritin is statistically significantly positively correlated to Gamma glutamyltransferase
Lactate dehydrogenase is statistically significantly positively correlated to Ferritin.
Lactate dehydrogenase is statistically significantly positively correlated to and Gamma glutamyltransferase.
Gamma glutamyltransferase is statistically significantly positively correlated to Ferritin.
Gamma glutamyltransferase is statistically significantly positively correlated with Lactate dehydrogenase.
In order to assess the utility of various parameters in identifying the abnormality the reference ranges are calculated by using Mean ± 2SD values of controls as shown in Table 9. The upper limit of this range is taken as the cut off values in identifying the abnormality.
Table 8
Reference ranges of various parameters
|
Parameter |
Reference range |
|
Ferritin |
4.69 – 180.24 µg/L |
|
Lactate dehydrogenase |
239.74 – 353.66 IU/L |
|
Gamma glutamyltransferase |
6.31 – 22.47 IU/L |
Ferritin identified % of cases as abnormal
Lactate dehydrogenase identified % of cases as abnormal
Gamma glutamyltransferase identified % of cases as abnormal
ROC Curve Analysis
In order to assess the maximum sensitivity, specificity of various parameters in identifying abnormality, the best cut off values are calculated using ROC analysis. Best cut off values are established by selecting a point closer to the left hand curve that provides greatest sum of sensitivity and specificity as shown in the Table 10.
Table 9
Sensitivity, specificity at best cut off value in discriminating between cases & controls
|
Parameter |
Best cut off value |
Sensitivity (%) |
Specificity (%) |
|
Ferritin |
> 158.1 |
100.0 |
95.56 |
|
Lactate dehydrogenase |
> 434.0 |
100.0 |
100.0 |
|
Gamma glutamyltransferase |
> 32.84 |
100.0 |
100.0 |
Diagnostic efficiency is defined as the portion of all subjects currently classified as having or not having disease.
Table 10
Diagnostic efficiency at best cutoff value in discriminating between cases and controls
|
Parameter |
Diagnostic efficiency % |
|
Ferritin |
97.7 |
|
Lactate dehydrogenase |
100 |
|
Gamma glutamytransferase |
100 |
Lactate dehydrogenase and Gamma glutamytransferase have shown the best diagnostic efficiency, followed by Ferritin at best cutoff value in discriminating between cases and Controls.
Area under curve
Area under curve provides unbiased estimates of sensitivity and specificity. It is a comprehensive representation of pure accuracy discriminating ability over the entire range of the test. It does not require selection of a particular detection threshold because the whole spectrum of possible detection thresholds is included.
Table 11
Area under curve for analyzed parameters in control’s and case’s
|
Parameter
|
AUC
|
P value |
95% CI |
|
Ferritin |
0.9985 |
< 0.0001 |
0.9951 to 1.002 |
|
Lactate dehydrogenase |
1.000 |
< 0.0001 |
1.000 to 1.000 |
|
Gamma glutamyltransferase |
1.000 |
< 0.0001 |
1.000 to 1.000 |
Lactate dehydrogenase and Gamma glutamyltransferase are the best discriminatory parameters in discriminating Breast carcinoma cases from controls followed by Ferritin.
Discussion
Breast cancer is the one of the most common carcinomas affecting women. There is an increase in Incidence of breast carcinoma world over. Even in countries with low incidence rates there are subsections of populations who are at increased risk of developing breast cancer. In India the incidence of carcinoma of breast is 23.2 per 100000 and there is a steady raise in incidence. Since breast cancer is potentially curable when diagnosed at early stages it is important to diagnose and initiate the treatment early. India which is still in low risk country category it has to be acted on promptly to prevent an emerging major increase of breast cancer in the 21st century.
Most cancers do not produce any symptoms until they have progressed to very late stages with metastasis to various organs. This is main cause for poor prognosis. Hence there is a need for detecting cancer at early stage. Various methods like Mammography, MRI, excision biopsy, FNAC are available and Tumour markers can be used for identification of at risk subjects, population screening, diagnosis and staging. However in our country these are not easily accessible and cost of investigation is also a crucial factor. Therefore there is a need for biochemical investigations which are economical and easy to perform.
In view of this the present study was undertaken to evaluate the role of Ferritin, Lactate de hydrogenase and Gamma glutamyltransferase in breast cancer patients and to assess their value in early detection, monitor therapy and progression. The present study was undertaken to evaluate the role of Ferritin, Lactate dehydrogenase and Gamma glutamyltransferase in breast cancer patients and to assess their value in early detection, monitor therapy and progression.
In the present study, the mean ± SD of serum Ferritin in the control group was 92.46 ± 43.89 and in the case group was 452 ± 174. The increase was significant (p < 0.0001). This study found that women with breast carcinoma, have higher concentration of Ferritin than normal healthy women. It is observed that greater levels of serum Ferritin concentration were seen patients with advanced stages of disease.
The findings of this study are reflected by the findings of Sandhya Mishra et al, Kher et al, Albuquer Que K.V. et al, Letiagin V. P. et al, Aydiner, A. et al, Kokocinaska D et al, who have shown that breast carcinoma patients have higher Ferritin concentration.
Albuquer Que K.V. et al, Letiagin V. P. et al, Aydiner, A. et al have shown that Serum Ferritin concentration was higher in cancer patients with metastases.
Asha kher et al correlated post treatment decrease in Ferritin levels with response to therapy, however persistent rise observed in few cases was attributed to recurrence of metastasis and concluded that Serum Ferritin is a good adjunct in the diagnosis, an indicator of stage of the disease, response to treatment and prognosis of the patient, and also serves as a tool in the early detection of local recurrence and metastases.
Ferritin has been considered as a useful diagnostic tool for early detection of breast cancer patients and Kokocinaska D et al compared its usefulness with CA 15-3 in patients of high risk.
Guner, G., Kirkali, G., et al have studied cytosol and Serum Ferritin in breast carcinoma and have reported elevated levels of Ferritin in breast cancer patients in their study.
Rise in serum Ferritin has been attributed to the iron requirement for cell growth and malignant cells requiring more iron to modulate transferrin receptor. Torti FM, Qi Y et al have proposed that hypoxia, often present in neoplastic tissue, is also one of the factor that promote Ferritin increase independently of iron status.
Elliot, R.L. et al documented transferrin receptors on proliferating and malignant cells in their cytochemical tissue culture and ultrastructural study on breast carcinoma and the role of iron metabolism.
Weinsein et al found that malignant tissue had six fold increase in the Ferritin concentration, when compared with nonmalignant breast tissue. Malignancies with the highest Ferritin concentrations were more anaplastic suggesting that the major site of the increased Ferritin was the malignant epithelium. They postulated that Ferritin may be a marker of neoplasia. Therefore studying Ferritin levels in breast carcinoma tissue may give us information about anaplasia and the proliferation index.
In the present study greater levels of serum Lactate dehydrogenase are seen in breast cancer patients. The increased levels of serum Lactate dehydrogenase in case group: mean ± SD 613.9 ± 168.6 compared to control groups mean±SD: 296.7 ± 28.48 were found to be significant (p values < 0.0001).
Also observed was the correlation between serum Lactate dehydrogenase values and stage of the breast carcinoma. There was a consistent increase as the disease progressed from stage I through stage IV.
Findings of the study were consistent with findings of a multitude of other studies which have shown elevations of serum Lactate dehydrogenase in breast cancer.
In a study conducted by Kher et al, Burke et al, Saravanan et al, R K Seth et al, increase in serum lactae dehydrogenase levels were observed in breast carcinoma patients.
The findings of this study are reflected by the findings of Sandhya mishra et al, who have shown that breast carcinoma patients with and without metastasis have higher serum lactae dehydrogenase levels.
Study done by Kharb et al and Nazoora khan in cases of carcinoma breast showed a steady stage wise increment of Lactate dehydrogenase as the malignancy progressed from stage I to Stage IV. Findings of the present study are consistent with these findings.
The cancer cells depend more on glycolysis, i.e., anaerobic respiration for their metabolism. For the regeneration of NAD+ the pyruvate produced at the end of glycolysis needs to be converted to lactate in a reaction catalyzed by Lactate dehydrogenase. Therefore, there is an up regulation of Lactate dehydrogenase in these malignant cells. LD-M is known to be regulated by activation of estrogen receptor in breast cancer cells, by protein kinase C mediated pathways. This could be a more specific cause for elevated lactate dehydrogenase levels in breast cancer. LDC/LDX an isoenzyme is shown to be present in malignant tissues, and the expression is similar to that of other germ-cell specific genes aberrantly expressed in cancer. This enzyme is restricted to cancer cells. Koukourakis et al studied differential expression of lactate dehydrogenase isoenzymes in breast cancer. P Khurana et al studied serum isoenzyme patterns in breast cancer. Lactate dehydrogenase also plays a role in the oxidant-anioxidant mechanism of tissues. It consumes NADH to produce lactate and thus contributes to oxidant damage. In cancerous tissues, due to excess growth large number of cells are destroyed and there is an increase in the levels of lactate dehydrogenase.
Liver is one of the frequent sites for metastasis of breast carcinoma. Cancers, which metastasize to the liver, cause a destruction of hepatocytes. Lactate dehydrogenase is a major liver enzyme and hence, its levels increase.
Therefore high levels of are seen in patients with metastasis, this is reflected in present study in concurrence with other studies like that of Konjevic et al. The greater dependence on glycolysis and excess cell destruction are the main causes for elevated Lactate dehydrogenase levels in serum of patients of cancer. Present study shows serum lactate dehydrogenase to be a useful marker for diagnosis of breast cancer, discriminating various stages, predicting prognosis and response to treatment in concurrence with previously done studies. In the present study, the mean±SD of Gamma glutamyl transferase was significantly higher p- value < 0.0001 in the cases group 60.64±16.24 that in the control group 14.39±4.04. This is in concurrence with other studies. Findings of the present study are similar to that of Sandhya mishra et al, Daniel F et al, and Viot M et al, who reported a significant increase in serum Gamma glutamyl transferase levels in breast cancer.
Correlation was observed between serum Gamma glutamyl transferase levels and the stage of breast carcinoma. There is a consistent increase as the disease progressed from stage I through stage IV. The results are in concurrence with the results of Kharb et al, in stage wise progression of serum Gamma glutamyl transferase values. The present study is in agreement with study of cant et al, who have shown that serum Gamma glutamyl transferase can be used for staging of breast carcinoma because of its high sensitivity and specificity. Coombes et al in their study of Biochemical markers in breast cancer, breast cancer management and medical complications of breast cancer have concluded that elevated serum Gamma glutamyl transferase is the best marker for detecting liver metastasis in breast cancer as the elevations start in very early stages. Gamma glutamyl transferase is one of the major liver enzymes. It is a very sensitive marker for hepatic dysfunction though not very specific.
It is present in the cell membrane and plays a role in the metabolism of Glutathione, and other thioredoxins and glutaredoxins, which are important antioxidant defence mechanisms. It is present in greater levels in breast tissue, and optimal activity is essential for proper milk protein production. Gamma glutamyl transferase provides cysteine to cancer cells in vivo, and this may be essential to metastasizing cells. Gamma glutamyl transferase is over expressed in some clones of breast cancer, and this may also be a cause for increased levels in certain cases. Dr. Tamaro proposed that in some instances the total levels of serum Gamma glutamyl transferase may be normal, but there is an elevation of Gamma glutamyl transferase 1 isozyme in breast cancer, which competes for the same receptors as estrogen and activates them, hence isoenzyme patterns can be studied for their utility in the diagnosis.
Conclusions
Breast cancer is the most common cancer among women world over and ranks second overall. It is now the most common cancer both in developed and developing countries. In India the incidence of breast cancer is increasing and it is the most common cancer in all urban areas and second most common in rural areas. It is amenable to cure if detected early. In the present study serum levels of Ferritin, Lactate dehydrogenase and Gamma glutamyl transferase were measured in breast cancer patients of various stages who formed case group and Healthy females constituted control group.