Introduction
The estimation of blood glucose levels is one of the most frequently ordered biochemical analyses. Accurate measurement of blood glucose concentration is especially essential for the diagnosis of diabetes mellitus.1 Current guidelines, proposed by the World Health Organization and American Diabetes Association, have defined the diagnosis of diabetes mellitus to be based on fixed cut points of blood glucose levels, even when characteristic symptoms of diabetes are present.1, 2 Due to such precise diagnostic criteria, the diagnosis of diabetes mellitus critically depends on accurate glucose measurements.
However, glucose is unstable in whole blood.1 The concentration of glucose in blood samples decreases rapidly at room temperature, largely because of glycolysis. This causes considerable error in measurements if some preservative is not included in the collection tube.3 Hence, the use of sample tubes containing sodium fluoride as an anti-glycolytic agent has become a widespread and established practice.
Although sodium fluoride is effective in minimizing glycolysis, the onset of this anti glycolytic action is not immediate. Fluoride has nil or minimal effect in the first hour, slows glycolysis considerably by the second hour and almost completely inhibits glycolysis by the fourth hour.4 This is because fluoride is an inhibitor of enolase (phosphopyruvate hydratase, E.C. 4.2.2.11), an enzyme far down in the glycolytic pathway, allowing enzymes upstream of enolase to remain active and metabolize glucose until the substrates are exhausted. Thus, this delay in the onset of anti-glycolytic action of fluoride reflects continuing metabolism of glucose despite inhibition of the downstream enzyme by fluoride.5 Therefore, it is logical to conclude that the use of sodium fluoride is justified only when the time interval between collection and assay is greater than 2-4 hours.4
Considering this, the guidelines for plasma glucose estimation (for the diagnosis of diabetes) recommend the use of plasma samples separated from cells within 60 minutes, and the use of sodium fluoride to inhibit glycolysis only when such separation is not possible.1 Other alternatives, such as icing6 and acidification7 have also been suggested. The use of a collection tube with a gel barrier that separates serum from cells (when centrifuged) appears to be another effective method to preserve glucose.7 Yet sodium fluoride continues to be used widely as the only glycolytic inhibitor in samples for glucose estimation.
We compare the fall in blood glucose levels in a sodium fluoride - potassium oxalate tube against the fall in glucose levels in a serum separator tube with a gel barrier and clot activator (SST tube) at various predefined time intervals. A heparinized tube without any agent to preserve glucose will act as a standard. The efficacy of the fluoride and SST tubes in preserving glucose will be compared with the heparinized sample as well as with each other, to determine the best method to preserve glucose, at different intervals from the time of sample collection.
Additionally, it has been reported that the packed cell volume (PCV, or hematocrit) values of the blood sample is a variable that significantly alters the rate of decrease of plasma glucose through glycolysis.8 Thus, this study also seeks to determine whether the hematocrit values of the blood samples have any correlation with the rate of glycolysis and the fall in blood glucose levels.
Aims and Objectives
The primary objectives of this study are to:
Compare the effectiveness of sodium fluoride in preserving plasma glucose against a serum separator tube (with a gel barrier), with a heparinized sample as standard.
Determine the more suitable method for glucose preservation at each time interval, keeping in mind the convenience and cost-effectiveness of each of the two tubes.
A secondary objective is to determine whether there is any correlation between the hematocrit values of the samples and the rate of glycolysis and the fall in the glucose levels, after the samples had been separated.
Materials and Methods
This prospective randomized study was carried out in a large tertiary care hospital and its associated clinical laboratory in south India. Approval from the institutional ethics committee was obtained and informed consent was obtained from all the participants. The samples were collected at the outpatient department and the analysis was carried out in the clinical biochemistry laboratory of the hospital, during the months of June and July 2013.
Sample collection and processing
45 samples were taken from volunteers at the blood collection site of the outpatient department. The inclusion criterion was any apparently healthy adult volunteer, and the exclusion criteria were patients on any chronic or regular medication. Both fasting and non-fasting volunteers were included. Cases of known diabetic patients were included, provided they were not under oral hypoglycemics. The samples were venous blood drawn from the ante cubital fossa or the dorsal venous arch of the hand. Around 5 samples were taken per day. Each sample was immediately distributed into four separate collection tubes directly from the syringe and the required number of inversions was carried out.
The first tube was a 3 ml lithium heparin (green top) tube, by AcCuvet-PLUS, Peerless Biotech Private Limited, India. The second tube was a 2 ml sodium fluoride - potassium oxalate (grey top) tube, by CB Plus, Quantum biomedicals, India. The third tube was a more expensive 3.5 ml serum separator tube (SST) with a gel barrier (gold top), marketed as SST II Advance, BD Vacutainer, Becton Dickinson and company. This tube has inert gel particles that form a barrier between the clot and the serum, thus preventing glycolysis. It also has walls coated with silica particles acting as a clot activator. The fourth tube was a 2 ml potassium EDTA (lavender top) tube, by CB Plus, Quantum biomedicals, India. This fourth tube was used for PCV analysis.
The tubes were immediately transported to the clinical biochemistry laboratory and all three tubes (except the fourth EDTA tube) were centrifuged to separate the cells from the plasma. The heparin and fluoride tubes were centrifuged at around 1,500 rpm in a swing bucket centrifuge for 2-3 minutes. The SST tubes, requiring a higher speed for the gel barrier to form, were centrifuged at 5,000 rpm for 5 minutes in a swing bucket centrifuge (after allowing 20 minutes for clot formation).
Laboratory analysis
The estimation of glucose was done via the enzymatic glucose oxidase / peroxidase method. Here, glucose is oxidized and peroxidase catalyses the formation of a pink colored complex, the intensity of which is directly proportional to the concentration of glucose in the sample. 1 ml of the reagent (from Coral diagnostics) was added to disposable test tubes, and 10 µl of the sample was pipetted in. After incubation at 37 ºC for 10 minutes in an incubator and development of the pink colour, analysis was done using a semi-automated colorimetric analyzer at 520 nm.
This same procedure was performed for estimation of glucose at all the time intervals of 1 hour, 2 hours, 4 hours, 12 hours, and on the following day, 24 hours. The pipetting and analysis for all the samples were performed by a single operator, the principal investigator of the study.
The fourth EDTA tubes were handed over to and independently analyzed by a staff member of the Institute of Pathology, using an automated blood analyzer. The values were reported and noted. All the tubes for all the samples were always kept at room temperature.
Statistical analysis
The means of the glucose values at each time interval was calculated separately for each of the three tubes. At each time interval of 1, 2, 4, 12 and 24 hours, an ANOVA (Analysis of Variance) was done on the combined set of all the measured values of all three tubes together. This yielded p values for the set of three tubes together at 1, 2, 4, 12 and 24 hours.
When the variation in glucose values of the group of three tubes at a particular time interval was found to be statistically significant, an ANOVA was done on the set of glucose values of each individual pair of the three tubes (Heparin-Fluoride, Heparin-SST, Fluoride-SST) at that time interval. When the variation in the glucose values of the group of three tubes at a time interval was not statistically significant, the mean of the differences in glucose values between the fluoride and heparin tubes (heparin subtracted from fluoride) at that time interval was determined and expressed as a percentage. This was to check if the difference in glucose values between the fluoride and heparin tubes was large enough to impact medical decision making (even when not statistically significant), by comparing with the criteria prescribed by the Clinical Laboratory Improvement Amendments (CLIA)(9).
The difference between the glucose values of each individual pair of three tubes was checked for correlation against the hematocrit values. This was done to analyse if a correlation existed between the PCV and the difference in the rate of fall of blood glucose levels in the three tubes.
Observations and Results
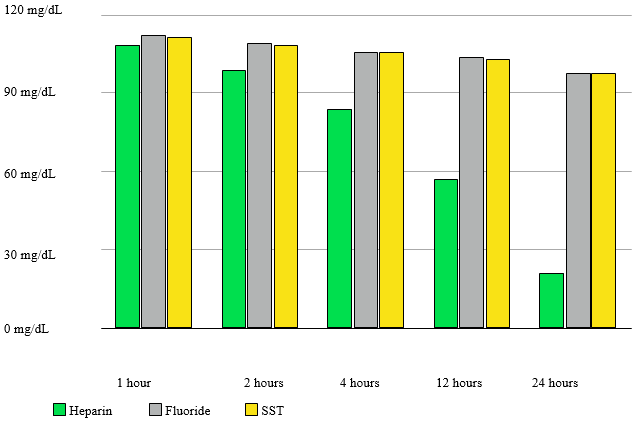
Figure 1 depicts the fall in glucose values at various time intervals by showing the means of the glucose values in each tube at each time interval.
Table 1 shows the results of a statistical analysis of the blood glucose values in each tube at each time interval. There is no statistically significant difference (p = 0.939, 0.605, and 0.0921 at 1, 2 and 4 hours respectively) in the glucose values measured in all the three tubes up until four hours. There is a statistically significant difference (p = 1.58E-05, 3.05E-15 at 12 and 24 hours) in the glucose values measured in the all the three tubes at the time intervals of 12 and 24 hours.
Table 2 shows the results of a statistical analysis of the blood glucose values measured in the three different pairs of tubes at 12 hours. There is a significant difference (p = 5.10E-05 for heparin and fluoride, and p = 6.19E-05 for heparin and SST respectively) between the glucose values in the heparin and fluoride and the heparin and SST pairs of tubes at 12 hours. There is no statistical difference (p = 0.9529) between the glucose values in the fluoride and SST pair of tubes at 12 hours.
Table 3 shows the results of a statistical analysis of the blood glucose values measured in the three different pairs of tubes at 24 hours. There is a significant difference (p = 2.99E-13 for heparin and fluoride, and p = 6.76E-13 for heparin and SST respectively) between the glucose values in the heparin and fluoride and the heparin and SST pairs of tubes at 24 hours. There is no statistical difference (p = 0.9880) between the glucose values in the fluoride and SST pair of tubes at 24 hours.
Table 4 shows the mean of the differences between the glucose values in the fluoride and heparin tubes at time intervals of 1, 2 and 4 hours. Based on CLIA criteria(9), there is an error beyond acceptable limits (accepted bias is 5% for glucose) when the glucose values measured in heparin and fluoride tubes are compared, at 2 and 4 hours (even though not statistically significant).
There was no correlation found between the difference between glucose values between the different pairs of tubes and the hematocrit values. The correlation coefficients (R - values) were less than 0.8 in all the cases at all time intervals.
Table 1
Statistical analysis of blood glucose values in all three tubes at various intervals
Table 2
Statistical analysis of glucose levels between individual pairs of tubes at 12 hours
|
Heparin and Fluoride |
Heparin and SST |
Fluoride and SST |
F crit |
|||
|
p - value |
F |
p - value |
F |
p - value |
F |
|
|
5.10E-05 |
18.15 |
6.19E-05 |
17.71 |
0.9529 |
0.003512 |
3.9493 |
Table 3
Statistical analysis of glucose levels between individual pairs of tubes at 24 hours
|
Heparin and Fluoride |
Heparin and SST |
Fluoride and SST |
F crit |
|||
|
p - value |
F |
p - value |
F |
p - value |
F |
|
|
2.99E-13 |
73.63 |
6.76E-13 |
70.70 |
0.9880 |
0.000226 |
3.9493 |
Table 4
Percentage difference in blood glucose levels between fluoride and heparin tubes at various time intervals
|
At 1 hour |
At 2 hours |
At 4 hours |
Acceptable error for a medical decision |
|
3.54% |
11.46% |
23.90% |
5.0% |
Discussion
The key finding of the study was that there was no statistically significant difference between the fall in glucose levels in either a fluoride tube or a serum separator tube with gel barrier at all time intervals. This means that there is no difference between a fluoride or a serum separator tube for glucose estimation, whatever be the time delay from collection to lab analysis. Hence, there is no need for the use of a separate fluoride tube for estimating plasma glucose when other analytes are also to be estimated; a serum separator tube can be used and all the analytes can be measured in the serum from the same tube. This minimizes the amount of blood collected from the patient and simplifies handling and processing of the blood samples.
However, if glucose estimation is the only test which is to be done for a particular patient, then the use of a fluoride tube would be more suitable as it has a considerably lower cost. It is also simpler to process, with separation taking place in a lesser time at a significantly lower centrifugation speed than a serum separator tube, which also takes a longer time for the gel barrier to form. Also, a serum separator tube has a 30-minute waiting time for the clot to form, and requires a larger volume of blood to be drawn. Finally, although there is likely very minimal difference between glucose estimation in plasma or serum,1 the use of plasma is still recommended for the diagnosis of diabetes.9
The findings that there was no significant difference between the three tubes until 4 hours, and no significant difference between the fluoride and serum separator tubes at all time intervals, echoes the results obtained by Li Geling et al.10 In this study, the experimental design was similar except they used a red top serum separator tube presumably without a gel barrier rather than a serum separator tube with a gel barrier (gold top). They concluded there was no significant difference between the three tubes until four hours and no significant difference between the fluoride and serum separator tubes for 4 days if refrigerated at 4 ºC. Thus, it can be concluded that there is no additional benefit in using a gold top tube with a gel barrier, which is contrary to what has been established by a previous study by Cuhadar, Serap, et al,11 which showed there was significant benefit in using a gel barrier in the estimation of glucose. However, in contrast to this study, Li Geling et al had stored the tubes at 4º C, which may explain the difference.
Since there was no statistically significant difference between all three tubes until 4 hours, it signifies that there is no advantage in using a fluoride (or SST) tube over a heparin tube for the first four hours. However, when the difference between the fluoride and heparin tubes were calculated, and the mean determined, it was found that the percentage difference between the glucose values in the heparin and fluoride tubes at 2 and 4 hours constituted an error beyond acceptable limits for medical decision making (according to CLIA criteria.12) Especially at the fourth hour, the glucose values in the heparin tubes were 23.9% lower than those in the fluoride tubes. Hence, it would be advisable to use a fluoride tube (or SST) for glucose estimation when the delay between collection and analysis is 2 hours or more. At the first hour, the difference was only 3.54%, and it can be strongly concluded that the use of a fluoride tube over a heparin tube does not confer any benefit if the delay between collection and analysis is less than an hour. This is confirmed by Chan et al, as they have shown that the fall in glucose levels in the fluoride tube parallels that of the heparin tube during the first hour.4
It can be concluded from this study that the use of a serum separator tube over a fluoride tube to prevent glycolysis during the first few hours does not confer any benefit. This may be because of the considerable waiting time of 30 minutes for the clot to form in an SST (as per manufacturer’s instructions). After the clot has formed, the tube must be centrifuged for 5-10 minutes, only after which the gel barrier is formed, and glycolysis is prevented. The time taken for transport, clotting and then centrifugation itself takes about an hour, during which period glycolysis is continuously taking place. Also, it may be possible that the process of clotting itself consumes glucose.7 When a fluoride tube is used, most of the glycolysis takes place only during the first hour, after which the rate of glycolysis slows considerably by the second hour.4 This may explain the similar fall in glucose values in both the serum separator tube and the fluoride tube during the first hour.
Attempts to prove a correlation between the difference in glucose values in the heparin tube (compared with the other two tubes) and the hematocrit were unsuccessful. Since it has been well established that the fall in glucose levels correlates strongly with haematocrit,8, 13 this contradictory finding may possibly be due to the smaller sample size.
Although this study suggests that there is no significant difference between a fluoride and SST, there are many studies that have concluded the exact opposite. Waring et al report a small but significant bias towards lower glucose concentrations in samples collected in fluoride oxalate tubes against a gel separator tube.14 This is of concern because diagnostic cut points for diabetes based on blood samples collected into tubes with NaF alone are likely to be too low,14, 15 increasing the risk of misclassification. These discrepancies in results may arise because of the sheer number of variables which affect glucose measurements, such as storage temperature, time when centrifuged and separated, serum - clot (or plasma - packed cell) contact time, the use of serum or plasma, methods of analysis, presence of interfering substances, choice of venous or capillary blood and probably hematocrit. Hence, more research, controlling for all these variables, is necessary to reach a consensus on the best method for glucose preservation.
Conclusions
A serum separator tube can be used when many analytes are to be estimated in the same sample, and a fluoride tube can be used when glucose is the only analyte to be estimated. It would be advisable to use a fluoride (or serum separator) tube if the time delay between collection and analysis is 2 hours or greater. A fluoride tube does not provide any benefit if the delay between collection and analysis is less than an hour. In such a case, a heparin tube can be used.
Further studies are needed to test the efficacy of the newer methods of glucose preservation (such as acidification) that are claimed to be more reliable16 when compared with traditional methods. It is imperative to reach a consensus on the most suitable method to preserve glucose and to adopt it universally, as the potential for diabetic patients to be misclassified and mismanaged because of this pre-analytical error is extremely large.17
Compliance with Ethical Standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional ethical committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Source of Funding
Partial financial support was received from the Indian Council of Medical Research (ICMR), under the short-term studentship program (2013-03022), to the corresponding author.
Conflict of Interest
The authors have no relevant financial or non-financial conflicts of interests to declare.