Introduction
The two most important forms of biologically inactive vitamin D are vitamin D3 and vitamin D2. Vitamin D3 is synthesized endogenously as a result of exposure of the skin to ultraviolet radiation with a wavelength of 290-310 nm. In this way, the synthesis of vitamin D3 is directly proportional to ultraviolet radiation exposure, inversely with the pigmentation of the skin.1, 2, 3, 4, 5, 6 In addition, factors such as geographical regions, atmospheric conditions, advanced age, the use of sunscreens, and the duration of light exposure also affect vitamin D3 synthesis.7 Vitamin D2, on the other hand, is produced by plants as a result of exposure to sunlight and is taken into the body by a diet containing plants.8, 9, 10, 11, 12, 13, 14, 7, 15, 16, 17 Vitamin D2 is found in small amounts in natural foods such as meat and egg yolks, mostly in fish. The amounts of vitamin D in some foods are given in Table 1. The precursor of vitamin D in plant tissues is ergosterol, and the precursor in animal tissues is 7-dehydrocholesterol.16 Since the way vitamin D2 and vitamin D3 are metabolized in the body and their potential for action is the same, they are both called vitamin D.
The History of Vitamin D
Since vitamin D photosynthesis has a history of 750 million years, vitamin D was first described as a vitamin in 1919-1920.5, 18 In his experiment on dogs, Sir Edward Mellanby found that rickets arises from a vitamin deficiency.18 In 1923, Goldblatt and Soames found that vitamin D is synthesized in the skin as a result of exposure of the precursor of vitamin D to sunlight.19 In Germany, in 1930, Windaus et al. found that vitamin D2 is produced from ergosterol and vitamin D3 from 7-dehydrocholecalciferol by ultraviolet rays.20 Initially, active vitamin D was considered a substance involved in calcium metabolism. The main actions, which include the absorption of calcium from the small intestine, the mobilization of bone minerals, and the reabsorption of calcium by the kidney, serve to increase the concentration of calcium in the blood and are necessary to successfully maintain the body's calcium level. Studies have revealed that VDR is found in tissues and organs all over the body, such as the skin, brain, and November, and is not limited to the small intestine, kidney, bone, and parathyroid gland.5
Chemical Structure of Vitamin D
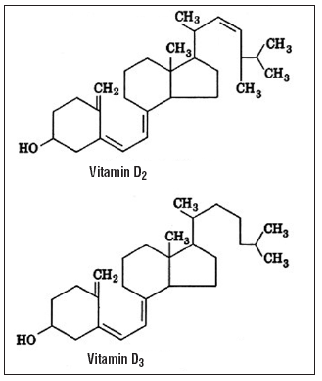
Vitamin D is similar in structure to sterol and it is a 27-carbon compound derived from the cyclopentanoperhydrophenanthrene ring. 3, 21 Vitamin D consists of four rings. The A, C, and D rings are saturated and have an 8 or 9-carbon side chain. In the B ring of the compound, 5 and 6 and 7 and 8 there is a double bond between the Decarbonates 9 to 10 the bond between the carbon has been Decoupled. The structure of vitamin D and the numbering of carbon molecules are shown in Figure 1.22
Vitamin D2 is Decoupled from vitamin D3 by having a double bond between carbon 22 (C22) and carbon 24 (C24) and containing a methyl group at carbon 24 (C24); this image causes the biological functions of vitamin D2 to be 3-10 times less than D3.21 The molecular structures of vitamins D2 and D3 are shown in Figure 2. 8
Cholecalciferol [25(OH)D3] has 3 double bonds. The melting point is 84-85°C, it does not have the property of dissolving in water. Its chemical formula is C27H44O3. 23
Vitamin D2 (ergocalciferol) is obtained from plants, especially mushrooms and yeasts. Structurally, vitamin D2 differs from vitamin D3 in that it has a double bond between C22 and C23 and a methyl group at C24.4 The deconstruction of vitamin D3 is not the same as that of C22 and C23. The chemical formula of ergocholesterol is C28H44O3.23
It is known that vitamin D has 37 metabolites, many of which are inactive. Among them there are metabolites such as 25,26(OH)2D, 1,24,25(OH)3D, 1,25,26(OH)3D, 25(OH)-26,23 lactone. The activities of bioliths have been determined.23 Vitamin D is metabolized to 25 hydroxyvitamin d (25OHD), then to the hormone form 1,25 dihydroxy vitamin D(1,25(OH)2 D).4
Metabolism of Vitamin D
Cholesterol synthesized in the liver is converted into 7-dehydrocholesterol, which is inactive provitamin D3, and reaches the Malpighi layer of the skin through peripheral blood. As a result of skin contact with the sun, high-energy ultraviolet rays (290-315 nm) pass through the epidermis, break down the double bonds in 7-dehydrocholesterol and form previtamin D3.2, 24
Vitamin D3 (cholecalciferol) is formed from vitamin D2 (ergocalciferol), and dehydrocholesterol (provitamin D3) from ergosterol, known as vegetable sterol, under the influence of ultraviolet (UV) rays. In the epidermis, Provitamin D3 is converted to previtamin D3 by UV-B radiation at a wavelength of 290-315 nm. Provitamin D is then isomerized to vitamin D, infiltrates the space outside the cell and dermal capillary vessels bind to the circulating vitamin-D binding protein (DBP) and are transported to the liver in this way. In the liver (through the enzyme 2 5-alpha-hydroxylase: CYP27) it turns into 25-OH-D (calcidiol). 25-OH-D is the measurement that best reflects the vitamin D stored in the body because it does not have biological functions at physiological concentrations. 25-OHD passes into the circulation and is transported from there to the kidney, undergoes 1-α hydroxylation in the kidney (via the enzyme 1-alpha-hydroxylase: CYP27B1), turning into the active metabolite 1,25(OH)2D3 (calcitriol).21, 25
Like all fat-soluble vitamins, vitamin D2, taken with food, and vitamin D3, taken from animal food and synthesized in the skin, are absorbed from the duodenum and jejunum in the small intestine under the influence of bile salts. After absorption, they pass from the thoracic duct to the bloodstream via chylomicrons (2,13,24,26). They bind to α-1 globulin, a vitamin D-Binding Protein (D-binding protein; DBP) in the blood and arrive in the liver. DBP is 1-3% free in the blood and 97-99% bound. DBP weighs an average of 53 kDa and the DBP gene is located on chromosome 4q11-13. The amount of DBP in plasma is approximately 20 times the amount of vitamin D and its metabolites present in circulation.12, 26
Vitamin D is supplied to the liver by the enzyme 25-hydroxylase 25. a hydroxyl molecule is added from its position and 25-hydroxyergocalciferol [25(OH) D2] or 25-hydroxycholecalciferol [25(OH) D3] is formed. The enzyme 25-hydroxylase is located in the mitochondrial or microsomes of hepatocytes. the 25-hydroxylase enzyme needs molecular oxygen, NADPH, and Mg to catalyze the reaction. This reaction is regulated by the feedback mechanism.12, 14, 24, 27, 28
25(OH)D, synthesized from vitamin D by the action of 25-hydroxylase enzyme and cytochrome p450 enzyme, is called ‘calcidiol’.14 25(OH)D is considered an important parameter reflecting the state of vitamin D in the organism. It is the main metabolite of vitamin D, and its half-life in circulation is approximately 3 weeks.14, 27
Calcidiol is found in the circulation due to DBP, only 1% of its total amount is in the free state in the circulation. In this way, a protective mechanism against vitamin D intoxication is formed.29 In addition, when high doses of vitamin D are taken, the plasma level of 25(OH) D3 increases, but some of the vitamin D taken is absorbed by adipose tissue, and thus not all of it is converted to 25(OH) D3.30
Calcidiol formed in the liver is re-bound to DBP in the circulatory system and comes to the kidney, which is the second most important organ in vitamin D metabolism. They bind to the megalin located on the membranes of the proximal tubulus cells of the kidney and pass into the cell. The mitochondria of these cells are rich in the enzyme 1α–hydroxylase. Here, calcidiol undergoes a second hydroxylation from the α position of the first carbon atom, turning into 1,25 dihydroxycholecalciferol [1,25(OH)D3] or 1,25 dihydroxyergocalciferol [1,25(OH)D2] (Figure 3). The new molecule formed is called 1,25(OH)2D ‘calcitriol’. 1,25(OH)2D is the most biologically active form of vitamin D.1, 12
Figure 3
Metabolism of vitamin D. a): Endogenous synthesis: vitamin D is 80% synthesized in the keratinocytes, after exposure to UVB light (290 to 315 nm), to convert the 7-dihydrocholesterol to pre-vitamin D3, and by thermal isomerization, cholecalciferol is generated; b): Factors influencing synthesis: melanin in the skin, extent of clothing, and topical sunscreen may generate less dermal vitamin D synthesis; c): Dietary sources: vitamin D can be obtained 20% from diet, cholecalciferol, also calcidiol and calcitriol in trace amounts are found in animal sources such as fatty fish, cod liver, offal, cheese and egg yolks, while ergocalciferol is found in yeast, sun-dried and ultraviolet irradiated mushrooms; d): Blood transport: ergocalciferol and cholecalciferol from diet or the cholecalciferol from endogenous synthesis passes into the bloodstream and binds to the VDBP for its transport; e): First hydroxylation: in the liver, ergocalciferol or cholecalciferol is metabolized by CYP2R1 to calcidiol, into circulation, calcidiol- VDBP is transported to the kidney; f): Second hydroxylation: calcidiol-VDBP complex is filtered through the glomerulus and endocytosed into the renal tubular epithelial cell, via megalin-mediated endocytosis or non-megaline-mediated uptake. In mitochondria, calcidiol is metabolized to the active form called calcitriol by CYP27B1 or is secreted directly to the interstitial fluid. Calcitriol can also be synthesized in other tissues where the CYP27B1 is expressed; g): Generation and excretion of other metabolites: stimulation of CYP24A1 produce inactive forms of vitamin D, such as 25(OH)D3-26,23-lactone, 24,25(OH)2D3 from calcidiol, 12,425(OH)3D3 from calcitriol, and finally calcitroic acid form is excreted in bile or urine; h): Intestinal calcium absorption: via VDR, calcitriol enhances intestinal calcium absorption in the small intestine, through the enhance the expression of the epithelial calcium channel TRPV6 and calbindin 9 K (CaBP); i): Parathyroid gland: when calcium plasma level decreases, PTH stimulates the production of calcitriol from calcidiol in kidneys. Calcitriol inhibits the PTH production and decreases bone resorption, increasing the urinary calcium excretion and FGF23 production by the osteocytes, leading to increased urinary phosphate excretion; j): Bone system: on the bone, calcitriol induces the expression of osteocalcin, and in osteoblasts via VDR, increase the expression of RANKL, that induces the bone mineralization; k): Others vitamin D targets: calcitriol can exert genomic actions others targets that express VDR such as cardiopulmonary system, brain, and immune cells. Vitamin D2: ergocalciferol; Vitamin D3: cholecalciferol; UVB: ultraviolet B; VDBP: vitamin D-binding protein; 24,25(OH)2D3: 24,25 dihydroxycholecalciferol; 12, 425(OH)3D3:1, 24, 25-trihydroxy vitamin D; VDR: vitamin D receptor; TRPV6: transient receptor potential cation channel, subfamily V, member 6; CaBP: calcium-binding protein; PTH: parathyroid hormone; FGF23: fibroblast growth factor 23; RANKL: receptor activator of the nuclear factor-jB ligand; RANK: receptor activator of nuclear factor-jB; Ca21: calcium; HPO4 22: phosphorus. GC, CYP2R1, CYP27B1, and CYP24A1 are the respective genes that encode the different proteins/enzymes involved in vitamin D metabolism.29

The circulating levels of calcitriol are a thousand times less than 25(OH)D and the half-life is 4-6 hours.31 25(OH)D can turn into 1,25(OH)2D in breast tissue, prostate, colon, and macrophages, especially in the placenta.12, 29 Ca and parathormone can strongly regulate renal 1α–hydroxylase and regulate it by the mechanism of 1,25(OH)2D feedback.32 the 1α-hydroxylase enzyme needs cytochrome p450, molecular oxygen, and reduced pyrimidine nucleotides (NADPH) to carry out the reaction. In addition, magnesium is a necessary parameter for the activation of the enzyme 1α–hydroxylase, as well as the enzyme 25-hydroxylase. Magnesium also increases the activity of adenylate cyclase during the formation of cAMP, which is necessary for the secretion and action of parathormones.12, 30
The activity of the extrarenal 1α–hydroxylase enzyme depends on the level of 25(OH)D and is not sensitive to parathormone (PTH) and extracellular Ca levels. While this situation increases the importance of 25(OH)D in the process of chronic diseases due to vitamin D deficiency, it causes the biologically active metabolite 1,25(OH)2D to be an undesirable parameter.32
Calcitriol, along with parathormone and calcitonin, plays an important role in the metacalcium and phosphorus metabolism 3). Active metabolites of Vitamin D exert their effects by binding to Vitamin D Receptors (VDRs) located in the cytoplasm and nucleus of target cells. VDRs have a hormone-binding part, a DNA-binding region, and an N-terminal region. These receptors are found in the kidneys, small intestine, parathyroid glands, osteoblasts in bone, islet cells in the pancreas, brain cells, circulating monocytes, activated T and B lymphocytes, gonads, and epithelium (Table 2). These tissues with VDR are also places that produce 1,25(OH)2D.15, 31 VDR is a 50 KD protein consisting of 427 amino acids and the VDR gene is localized on chromosome 12q13-14.2
Table 2
Cells with VDR in the cytoplasm, nucleus, and membrane.33
Serum Vitamin D Levels
Since vitamin D is a fat-soluble vitamin, it is stored in the liver and adipose tissue. That is why, for up to 6 months, when there is a deficiency or absence in dietary intake, the body can meet its own needs. On the contrary, in obese people, vitamin D deficiency may occur, as the rate of stored vitamin D will also increase due to the increased fat content.34
It has also been noted that VDR polymorphism increases the frequency of Hashimoto's thyroiditis. Hashimoto's thyroiditis has been found in studies in which vitamin D deficiency is observed in patients.34
Even though the active form of vitamin D is 1.25(OH)2D, the parameter that gives the most accurate result about its level in the body is 25(OH)D. The reason for this is that the half-life of 1,25(OH)2D is 3-6 hours, and the half-life of 25(OH)D is 20 days. Since 25(OH)D remains in the body for a longer period, this metabolite is measured in the diagnosis of vitamin D deficiency.12 In addition, 25(OH)D is found in the body 1000 times more than 1.25(OH)2D. The normal values of 25(OH)D and 1.25(OH)D2 and 1.25(OH)D3 are given in Table 3.23
Table 3
Normal values of vitamin D metabolites8
|
Metabolites of Vitamin D Normal Values |
|
|
25(OH)D2 Infant |
4-10 ng/mL |
|
25(OH)D3 Childhood |
12-40 ng/mL |
|
25(OH)D Adolescent |
30-50 pg/mL |
|
24.25(OH)D3 Adult |
1-4 ng/mL |
In infants, vitamin D is obtained through the synthesis in the skin by placental passage, breast milk, and sunlight. It is reported that the serum 25(OH)D levels of infants in the first eight weeks of life are related to those of their mothers, and in the following months, sunlight becomes more decisive.11 It is believed that the most critical risk factor for vitamin D deficiency or insufficiency in early infancy is vitamin D deficiency in the mother.10
There is no complete consensus on the reference range of vitamin D. In most laboratories, the upper limit for the level of 25(OH)D is considered to be about 50 ng/ml. For vitamin D excess, a 25(OH)D level higher than 150 ng/ml associated with hypercalcemia is diagnostic. In addition, it is known that vitamin D is not life-threatening even at levels of 100 ng/ml formed by sunbathing, and poisoning does not occur.35
Holick MF et al. in their research obtained a normal value by comparing the level of vitamin D with the level of parathyroid hormone. In this study, the threshold value of parathyroid hormone was taken into account and the amount of vitamin D at that time was examined. Holick MF et al. measured the amount of vitamin D corresponding to the plateau-drawing parathyroid hormone as 32 ng/mL and accepted this value as a normal value. Accordingly, the values between 21 and 29 ng/mL were evaluated as insufficient and the values below 20 ng/mL were decisively assessed.36
Bischoff-Ferrari et al. on the other hand, bone mineral density (BMD), lower extremity functions, dental health, fracture formation, and colorectal cancer formation were taken into account by evaluating serum 25 (OH)D levels, and the best results were obtained when 30 ng/ml (75 nmol/L) was exceeded.37
Kidney dialysis Outcomes Quality Initiative according to the manual, circulating 25(Oh)D3 Level 5 ng/mL is lower than if severe vitamin D deficiency 5 to 15 ng/mL vitamin D deficiency is mild, 15 - 29 ng/mL vitamin D deficiency, 30 mg/mL is higher than normal vitamin D levels to 150 ng/mL higher than vitamin D intoxication is evaluated as.38 According to the evaluation of the Centers for Disease Control and Prevention CDC (Centers for Disease Control and Prevention CDC), vitamin D levels are given in Table 4.12
Table 4
Interpretation of serum 25(OH)D value12
In the circular vitamin D levels, it is accepted that below 25 ng/ml is insufficient and below 10 ng/ml is considered a deficiency. In adults, the desired level is above 40 ng/ml.12
The Need for Vitamin D
There is no clear consensus on the need for vitamin D, as well as on its levels in the serum. The fact that this situation is still a subject under discussion has the effect of varying the degree of contact of the skin with the sun's rays. In recent years, it has been concluded that the need for vitamin D in children has generally increased. According to the World Health Organization (WHO), the daily requirement of vitamin D for infants is 400 units.12, 16
The U.S. Food and Nutrition Board until the age of 50 and 200 U/day for those aged 51-70 years, 400 U/day, the next age 600 U/day of vitamin D recommends an intake (39). The American Academy of Pediatrics recommends vitamin D 400 U/day up to the age of 1, 600 U/day for all men and women from the age of 1 to the age of 70, and 800 U/day for those over the age of 70 in the vitamin D dietary guidelines published in March 2011. The Ministry of Health of Canada recommends 400 U of vitamin D daily for all children up to the age of one, 600 U daily for those aged 1-70, pregnant and lactating mothers, and 800 U daily for those older than 70 years (Dec 11).
The Ministry of Health of Turkey, the Prevention of vitamin D deficiency and bone health in infants under the protection of the decision on the project from the first week of May 2005 birth, regardless of diet (formula or breastmilk, it doesn't matter) of all babies up to the age of at least one, preferably 3 years old up to 400 U/day of vitamin D recommends that it be given.12
In addition, according to the circular of the Ministry of Health published on May 9, 2011, it was planned to provide Vitamin D support to mothers for a total of 12 months, including six months during pregnancy and six months after childbirth, starting from the age of 12 weeks.12
In pregnancy and lactation, the optimal vitamin D requirement is unknown. But it is observed that it is higher than the reference values, which vary between Dec 200 and 400 U/day. Compared to pregnant women who received and did not receive vitamin D support of 1000 U / day in the last trimester of pregnancy, it was found that babies born from pregnant women who did not receive vitamin D had more intrauterine growth retardation, babies gained more weight when they turned one year old, and the growth rate was lower.39 Hollis B.W. and Wagner C.L. report that by giving 2000 and 4000 U /day of vitamin D to nursing mothers, the vitamin D needs of infants receiving breast milk will be met.40
Mechanism of Action of Vitamin D
Vitamin D shows its effect with its active metabolite, 1,25(OH)D. 1,25(OH)2D binds non-covalently with intracellular receptor proteins found in almost all tissues.41, 42
The effect on the receptors is in the form of a genomic effect or a non-genomic effect, as is the case with all steroid hormones.41
The Genomic Effect of Vitamin D
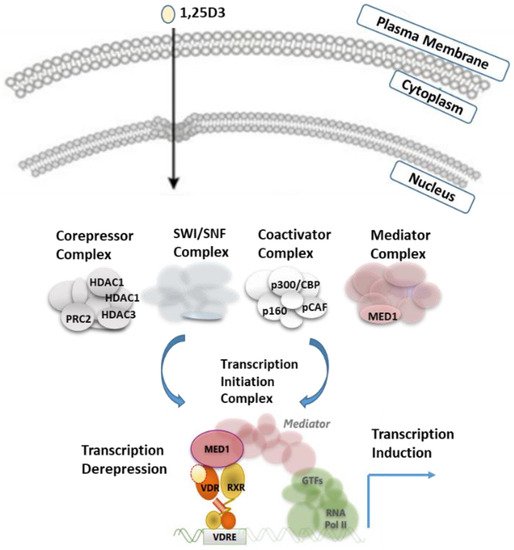
The genomic effect is in the form of gene transcription via nuclear VDR, which occurs directly within hours or days. The formation of vitamin D and its effect on transcription is shown in Figure 4. Vitamin D is known to keep more than 200 genes under control. Almost all gene expression studies of vitamin D emphasize that active vitamin D directly or indirectly regulates 0.8-5% of the total genome. This active vitamin D is in the regulation of cellular growth, DNA repair, differentiation, apoptosis, membrane transport, cellular metabolism, and adhesion, and describes taking part in many events, such as oxidative stress.41
CYP2R1 is the most important 25-hydroxylase; CYP27B1 is the key 1-hydroxylase. Both 25OHD and 1,25(OH)2D are catabolized by CYP24A1. 1,25(OH)2D is a ligand of the vitamin D receptor (VDR), a transcription factor, and binds to regions in DNA called vitamin D response elements (VDRES). There are thousands of these binding sites that regulate hundreds of genes in a cell-specific way. Transcription regulated by the VDR depends on commodities, the profile of which is also cell-specific. Analogs of 1,25(OH)2D are being developed to target specific diseases with minimal side effects.4
Non-genomic Effect of Vitamin D
The non-genomic effect occurs via VDR on the cell membrane, which occurs in a shorter time, such as minutes. This non-genomic effect is realized by changing the calcium-chloride transmembrane transition of ions, which are usually transient, or by activating intracellular signaling pathway activities (cAMP, PKA, phospholipase C, PI-3 kinase, and MAP kinase).41
Genetic changes in the VDR gene cause changes in the protein sequence, and thus important problems can arise that affect cell proliferation and immune functions, as well as calcium metabolism. On the other hand, active vitamin D can also act on calcium channels, beta cells of the pancreas, vascular smooth November muscles, intestines, and monocytes by activating secondary messengers such as MAP or cAMP by binding to the plasma membrane receptor (non-genomic effect).29, 41
Vitamin D genomic and non-genomic effects in addition to 1,25(Oh)2D skeletal muscle, immune system, and nerve growth factor (NGF-nerve growth factor) that has a regulatory role in cell differentiation and reactivate proteins, such as neurotransmitters in the central nervous system that act as vitamin D deficiency and diabetes, tuberculosis is a risk factor predisposing to coronary heart disease and it is suggested that.10, 44
Regulation of Vitamin D
Since vitamin D acts as a hormone, its levels in the organism are adjusted by a feedback mechanism. In addition, vitamin D can act on many tissues and cells by binding to a membrane receptor on plasma and affecting secondary messengers, which are cAMP and MAP. The formation of vitamin D and the mechanism of its action are shown in Figure 5. The key enzyme in the mechanism of vitamin D is the enzyme 1α-hydroxylase. PTH, phosphorus, calcium, and fibroblast growth factor 23 (FGF23) play an active role in the regulation of the enzyme 1α–hydroxylase.12, 23, 45
The regulation of vitamin D is divided into 4 parts;
When calcium and phosphorus levels decrease in serum, the enzyme 1α-hydroxylase is activated by stimulation and increases the synthesis of vitamin D.23
Parathormone increases the synthesis of vitamin D by stimulating the enzyme 1α–hydroxylase in tubulus cells in the kidney.23
When the active metabolite of vitamin D, 1,25(OH)2D, reaches sufficient levels in tissues, the enzyme 24-hydroxylase becomes active and binds to 1,25(OH)2D with the help of a feedback mechanism, inactivating it. In this way, vitamin D is catabolized and the excess of the inactive metabolite formed is excreted through the biliary tract. On the other hand, when the amount of 1,25(OH)2D decreases, the enzyme 1α–hydroxylase increases, while the enzyme activity of 24–hydroxylase decreases.12