Introduction
In tropical and subtropical areas, filariasis is a persistent illness that causes significant morbidity. Though the World Health Organization's mass medication program has assisted in reducing the prevalence of filariasis, to control and lower the number of microfilariae infections in the human population, however, more research will be required on fresh medications and vaccines.1 Setaria digitata is a filarial parasite that causes lethal cerebrospinal nematodiasis in goats, lambs, and horses, resulting in enormous economic losses in the tropical animal husbandry industry. Because of its similarities to Wuchereria bancrofti, this worm is frequently used as a model organism in the study of the mechanism of human lymphatic filariasis for drug development.2 Anti-parasitic drugs may act by combating the disease-causing organism or by altering the host variables that cause the parasite to die.3 Diethylcarbamazine (DEC) is one such drug that is effective in the treatment of W.bancrofti infection in humans. Experiments showed that DEC administration rapidly caused microfilaremia and microfilaruria with a rapid reduction of microfilariae in the peripheral blood. At the same time, DEC caused several side reactions, particularly in the first 1-2 days of administration. The reactions included specific allergic reactions such as high fever, swelling of lymph nodes, fatigue, etc, and toxic reactions such as headache, lumbago, loss of appetite, and nausea.4 Though the effectiveness of DEC against lymphatic filarial is known its effect on the antioxidant enzyme activities of the host mice infected with microfilaria is not well studied. The objective of this study was to evaluate the effect of DEC on the antioxidant enzyme activities, superoxide dismutase (SOD), and catalase (CAT) in mice infected with S.digitata microfilariae (Mf).
Materials and Methods
Collection of parasite and microfilariae (Mf)
S.digitata from the peritoneal cavity of Bos indicus were collected in Tyrode medium (NaCl 0.8%; KCl 0.02%; CaCl2; MgCl2; NaHCO3 and Glucose 0.5% 5 form the Government of Kerala authorized local abattoir. The medium was used to wash away the extraneous adhering from the parasite's surface. After that, the worms were maintained in Tyrode medium at 370C until needed. S.digitata was incubated in the Tyrode medium at 370C for two hours and was centrifuged to collect the Mf.
Chemicals and animals
Highly pure chemicals and reagents purchased from Sigma Chemicals Co, USA were used for the present study. Swiss strain inbred mice that were 30 days old and weighed 10–12g were used. Each experiment used a maximum of 6 animals. Additionally, adequate controls were kept. The institutional animal ethical committee's guidelines were followed in all animal research.
Infection
Four groups of mice were used, and Figure 1 shows the treatment schedule for each group. Diethyl ether was used to anesthetize mice after a 12-hour fast, and they were restrained by adhering posters to the dissection board. A sterile syringe was used to inject penicillin and streptomycin-containing PBS suspension of around 5000 Mf into the ventral peritoneal cavity. Mice used as controls received injections of PBS laced with Mf with streptomycin and penicillin.
Administration of drug
Since Diethylcarbamazine (DEC) is thought to be effective against infections from W. brancrofti and B. malayi, it was given orally to the experimentally infected mice at a dose of 300 mg/kg bwt/day for 12 days. (Hawking 1978). Animals were euthanized and the peritoneal fluid and organs were removed and stored immediately for further examinations.
Antioxidant enzyme levels
Superoxide dismutase (SOD) activity
SOD activity was evaluated in the peritoneal fluid, kidney, liver, and spleen of DEC-treated and Control mice using the protocol described by Nishikimi et al.6 The reaction was initiated by adding NADH to the assay mixture, which also included water in a volume of 3 ml, 1.2 ml of sodium pyrophosphate buffer (pH 8.3; 0.052M), 0.1 ml of 186 mM PMS, 0.3 mM NBT, and 0.2 ml of 780 mM NADH and roughly diluted enzyme preparation. After 90 minutes at 300C of incubation, the addition of 1 ml of glacial acetic acid stopped the reaction process. 4 ml of n-butanol was added to the reaction mixture and mixed briskly before being let to stand for 10 minutes. The layer of butanol was removed after centrifugation. At 560 nm, the chromogen colour intensity in the butanol layer was measured. All reagents without enzymes were used as blank.
Catalase (CAT) activity
CAT activity was evaluated in the peritoneal fluid, kidney, liver, and spleen of DEC-treated and Control mice using the protocol described by Luck et al.7 The reaction mixture contained Phosphate buffer 0.07 M; pH 7.0; H2O2-0.07M phosphate buffer (.16 ml H2O2 diluted with 100ml buffer). The OD of the solution should be about 05 at 240nm. Samples were read at 240 nm against the control. The time required for the decrease in OD from 0.45 to 0.40 was noted and used for calculation.
Results
Effect of DEC on the SOD and catalase activity
Figure 2
Effect of DEC on the Superoxide Dismutase activity in the Peritoneal fluid, Liver, Spleen, and Kidney of the control, DEC, Control+Mf, and Control+DEC+ Mf treated mice.Values are expressed as ± SD of three independent estimations. A: SOD level in the peritoneal fluid; B: SOD level in the spleen; C: SOD level in the Kidney; D: SOD level in the Liver. ;*Significant compared to the control (p<0.05); **Significant compared to Control+Mf (P<0.05).
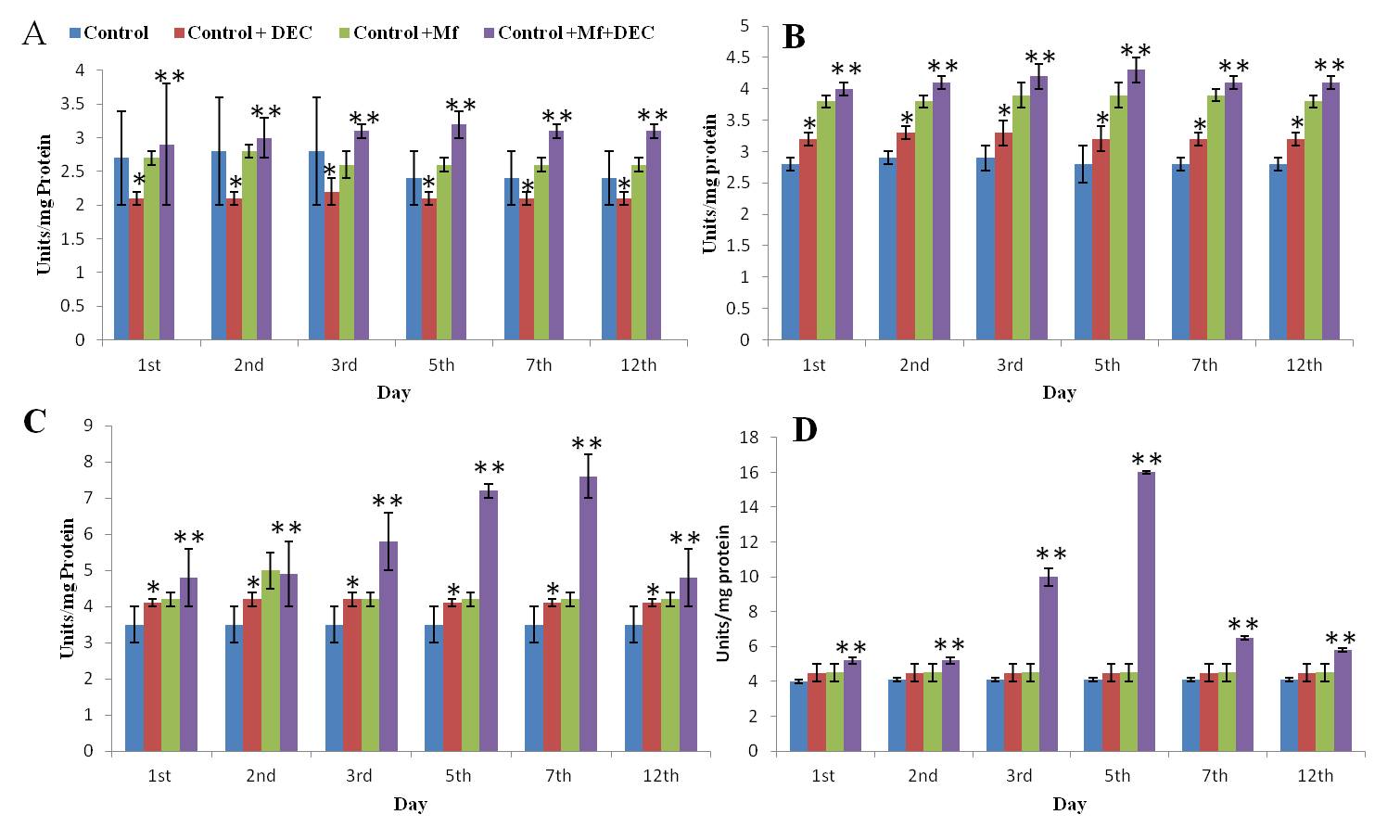
Figure 3
Effect of DEC on the Catalase activity in the Peritoneal fluid, Liver, Spleen, and Kidney of the control, DEC, Control+Mf, and Control+DEC+ Mf treated mice.Values are expressed as ± SD of three independent estimations. A: CAT level in the peritoneal fluid; B: CAT level in the spleen; C: CAT level in the Kidney; D: CAT level in the Liver. ;*Significant compared to the control (p<0.05); **Significant compared to Control+Mf (P<0.05).
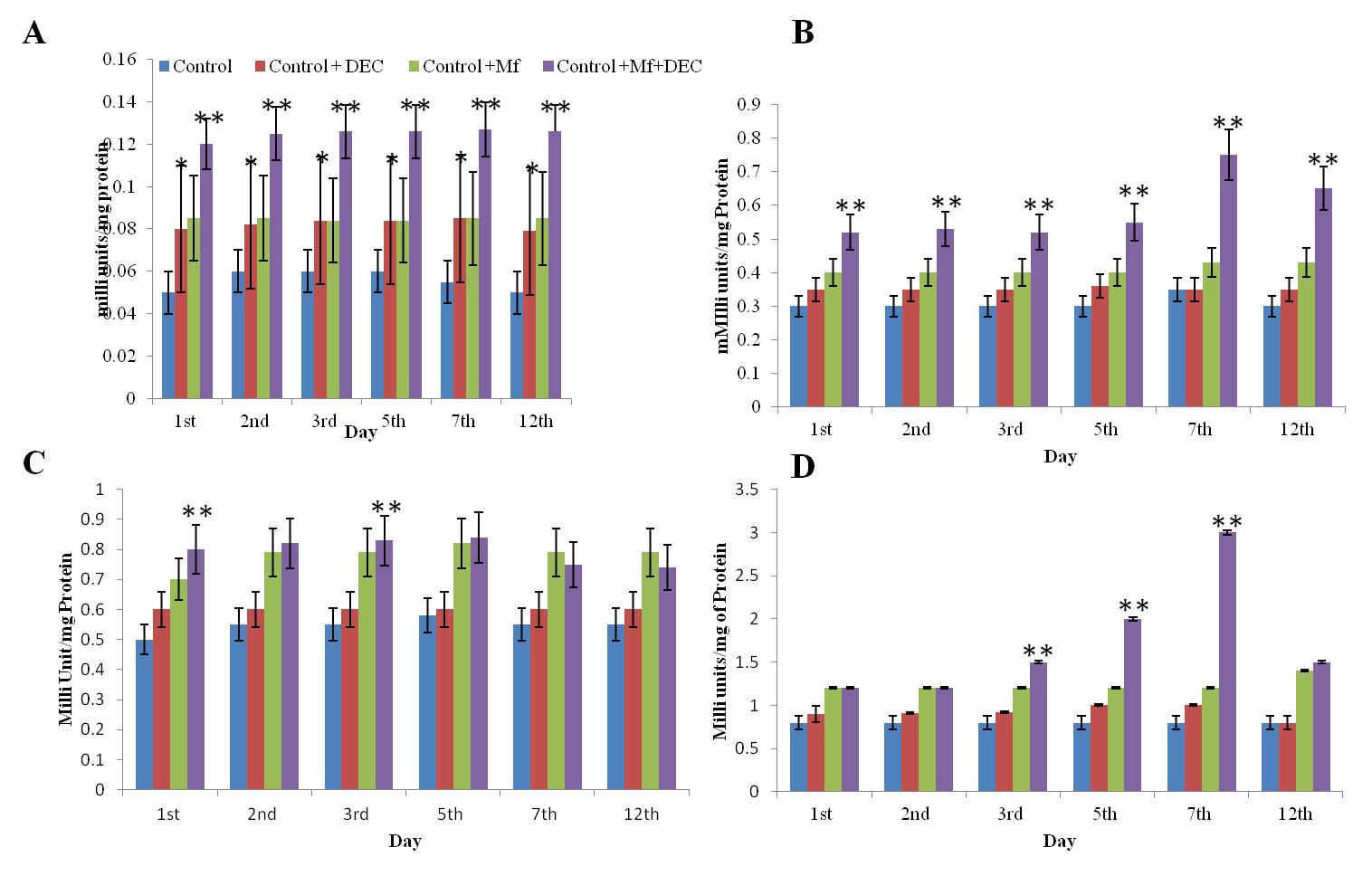
Levels of SOD and CAT were estimated in the liver, kidney, spleen, and peritoneal fluid of mice in mf+ve, DEC treated, and normal. In the peritoneal fluid, CAT activity in the peritoneal fluid of Control+Mf+DEC was found to be elevated at a higher level compared to the control. The activity in the Control+DEC and Control+Mf mice showed similar values. SOD activity in the peritoneal fluid of Control+Mf+DEC mice remained elevated from the 1st day to the 12th day compared to the control. Unlike CAT, the SOD activity in the peritoneal fluid of Control+mf mice was found to be higher than in Control+DEC animals. In the Liver and Kidney Liver and kidney showed significantly altered levels of SOD and CAT in test groups compared to controls (Figure 2, Figure 3). In the liver, the SOD level was significantly high, especially during the 3rd and 5th day and it was found the level gradually decreased and reached the normal level on the 12th day.
Discussion
This investigation was started to determine how DEC affected S. digitata Mf-infected mice as compared to normal control, control + Mf, and control + DEC. The generation of active oxygen species, which is thought to happen as a result of a tilt in the metabolic balance of the cells towards oxidative damage, may be the cause of the elevation in SOD levels observed during the third and fifth day in DEC-treated Mf-infected mice.8 Activated phagocytes produce more free radicals in response to infection.9 The enzymes SOD and CAT, which catalyze the formation of hydrogen peroxide to form superoxide anions and act to decompose hydrogen peroxide, are a major protective mechanism for copying with free radicals. The adult Setaria worms taken from the peritoneal cavity of cattle when injected intraperitoneally in mice, Mf appeared in the blood and persisted for some days. These Mf were found to be sensitive to different doses of DEC.10 When DEC was administered orally to either animal or man, it is absorbed from the alimentary canal. In mice, it is absorbed and rapidly distributed, reaches the liver, kidney, adrenal glands, muscle, and gastrointestinal tract and accumulates in the brain in 20 min, and diminishes after one hour.11 Metabolism of the drug is also very rapid and is excreted in four different forms, in all of which the piperazine ring remains intact no abortifacient or teratogenic effect is reported.12 Certain reactions to treatment with DEC occur in persons infected with Bancroftian and Brugian filariasis. These are thought to be immunological reactions to the disintegrating mf and dead adult worms.13 It has been shown that its microfilaricidal action depends entirely on damages caused by the oxygen radicals, and this may subsequently lead to activation of the immune system of the host. Thus, initially, mf from the blood is destroyed by the reticuloendothelial cells of the liver, and once activated, the immune system takes care of the further destruction of the mf. Reports also show that DEC induces DNA fragmentation, a characteristic of apoptosis in microfilariae of Wuchereria bancrofti 14 DEC, which also shows anti-inflammatory activity by inhibiting cycloxygenase and lipoxygenase, 15 caused significant cellular disorganization in microfilariae cells, resulting in an abundance of degenerating organelles, large and numerous vacuoles, and nuclear condensation. Despite the loss of cellular substance in certain cells, the plasma membrane retained its integrity. All of these characteristics revealed DEC promotes apoptosis in W.bancrofti microfilariae.16, 17, 18 Treatment with Oleanolic acid from anti filarial triterpene saponins of Dipterocarpus zeylanicus, showed significant oxidative stress in S.digitata as evidenced from the decreased levels of reduced glutathione (GSH) and elevated levels of glutathione S transferase (GST), superoxide dismutase (SOD) and reactive oxygen species (ROS).19 Microfilariae, due to the apoptotic action of DEC may secrete several molecules which challenge the immune system of the host resulting in inflammation and free radical production, which may be one reason for the elevetaed levels of SOD and CAT enzymes.
The present study showing a distinct delayed effect suggests that there are still factors remaining to be understood in the mechanism of action of DEC against filarial parasites and in filariasis. The result obtained in the present study opens up that sequence of information.