- Visibility 76 Views
- Downloads 21 Downloads
- Permissions
- DOI 10.18231/j.ijcbr.2022.019
-
CrossMark
- Citation
Coronavirus disease 2019 in diabetes: A pathophysiological linkage
- Author Details:
-
Shreshtha Gaur
-
Surabhi Bajpai *
-
Sonal Gaur
-
Sonu Singhal
-
Rakesh Mishra
Abstract
Coronavirus disease 2019 (COVID-19) specifically in diabetic patients has attracted attention worldwide due to the poor prognosis of infection, compromised immunity and delayed response to medicines leading to increased death rate. Several pathophysiological explanations can be linked in support of connection between severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) alias COVID-19 and diabetes severity. In patients with diabetes, the innate immune system is compromised and the disease can be triggered by SARS-CoV-2. The exaggerated and inappropriate cytokine response can be evidenced in both diabetic and COVID-19 patients. This is evidenced by the elevated levels of IL-6 in their blood. It has been known that people with diabetes are more prone to having an inflammatory cytokine storm, which can cause acute respiratory distress syndrome (ARDS). Anti-viral drugs and agents can help lower blood sugar levels, but their use should be carefully monitored to see if they can also interact with COVID-19 treatment.
Introduction
The outbreak of COVID-19 affected various countries and different parts of the world. It was brought by the SARS-CoV-2 virus which results in increased number of deaths each day. COVID-19 started spreading globally in December 2019. Initially the cases of COVID-19 were reported in Wuhan, China. Quickly it reached to other countries and affects their population. Large number of front-line medical personnel in Wuhan were affected by this lethal virus. Around 3019 medical staff members in Wuhan were infected with COVID-19, which led to 5 deaths.[1] Diabetes is another global long-standing non-infectious metabolic syndrome which is evidenced to affect 463 million adults in 2019 and reports for 9.2% of the population globally aged 29-79 years.[2] Several reports have evidenced that diabetic individuals are at increased risk of COVID-19. According to a study, it has been revealed that among the patients with COVID-19, 32% had diabetes, 16% had vascular disease, and 15% had hypertension. In 2019, it was estimated that the cost of treating type 2 diabetes globally reached approximately 760 billion dollars. It led to the deaths of about 4.2 million individuals. [3] People with diabetes who were diagnosed with COVID-19 have a higher mortality rate than those deprived of the condition. This conclusion was made after a study revealed that the viral infection caused by COVID-19 led to higher mortality rates. The pandemic has been managed to control its spread while protecting the general population. While it is true that individuals with diabetes are considered vulnerable, it is worthy to note that they should not be ignored.[4] According to a survey done in England (UK) it was found that out of the 23804 patients who died due to COVID-19, 32% had diabetes and 15% had type 1 diabetes. The study revealed that these patients had a higher chance of dying than those without diabetes.[5] It is important to maintain the safety of the diabetes population and minimize the risk of developing diabetes-related complications. Moreover, linking the two diseases by their signaling pathways may unveil key molecular players that maybe helpful in future therapeutics. The present study aims around providing an overview of the link between COVID-19 and diabetes by possible pathways involving them. Most of the information discussed in this review pertains to patients with type 2 diabetes and focuses on patients with type 2 diabetes until unless specified.
Etiology
In China, on 29 December 2019, the first case of an acute respiratory distress syndrome (ARDS) was reported among people living locally in Wuhan City, who were associated to a nearby seafood marketplace. It has been observed that maximum previous cases were also connected to the original seafood market.[6] In no time, it was found that the outbreak was caused by a secondary source of infection that originated from close contact with infected individuals. The number of cases in the city increased between people infected with the disease who had no prior connection to wildlife. Multiple positive cases for this contagious disease were detected among hospital staff personals and medical professionals. It was later identified that the infection spreads via exposure to the virus which later found to be SARS-CoV-2. By the time the virus was detected, it had already come into the human chain and esclated at an alarming rate affecting many countries worldwide. It was suggested that the most at-risk individuals were those with weak immune systems, such as those with chronic kidney and liver dysfunction. Infected patients aged among 25 and 89 years old usually fall under this age distribution according to some reports. The cases among infants and children were initially less severe.[7]
Diabetics and Cytokine Storm
The innate and humoral immunity are compromised in the diabetic patients who have uncontrolled glycaemic levels. Diabetes can cause a proinflammatory state which leads the upregulated cytokine response with increased level of interleukin-6 (IL-6), also reported in COVID-19.[8] Entry of SARS-CoV2 into a host cell activates an inflammatory response that produces an army of interferon gamma (IFN-γ) which results into a cytokine storm.[9] One of the key factors that trigger the process of pathogenesis is cytokine storm which ultimately results in plasma leakage, disseminated vascular coagulation and permeability of vascular membrane detected in COVID-19 patients.[10] The initiation of cytokine storm is an uncontrolled immunologic response resulting in constant expansion and activation of macrophages, immune cells and lymphocytes. The response generates massive amounts of cytokines, consequently forming a cytokine storm. Increase in the level of the proinflammatory cytokines like IL-1, IL-6, IL-18, IFN-γ, and TNF-α is the hall mark of cytokine storm.[11]
Diabetics have shown a downregulation in the expression of angiotensin-converting enzyme 2 (ACE2). The enzyme is known to found in multiple organs including lungs, kidneys, pancreas, vascular system and intestinal endothelium. Interestingly, ACE2 is known to play a key role in the anti-oxidation and anti-inflammation activities of the body. It is also responsible for the degradation of various angiotensin-II components, namely angiotensin (1–7) and angiotensin (1–9) respectively as well as to a lesser extent to angiotensin I. Angiotensin 1–7 is known to responsible for the anti-inflammatory and antioxidant effect. This process is eventually compromised in pathophysiology of diabetes. If the diabetic patients are infected by COVID-19, they are at increased risk of suffering from severe lung injury as well as ARDS. [12] The above discussion signifies that the imbalance in ACE 2 activation pathways results in upregulation of levels of angiotensin II and a downregulation in the level of angiotensin 1–7. [13] Albeit, expression of ACE2 also occurs in pancreas, therefore during the virus entry into the pancreatic islets, it may cause beta-cell dysfunction resulting hyperglycemia as fate.[14] In the end, the cellular mechanisms/pathways generated by COVID-19 and the pathophysiology of diabetes, diabetics are more prone to cytokine storm with high potentiality of organ damage if infected by COVID-19.
COVID-19 Triggered Pathways and their Possible Links with Diabetes
The binding of the viral spike protein of infective SARS-COV-2 to the host cells occurs with the help of entry receptor ACE2. The single stranded viral RNAs, are detected via pattern recognition receptors as pathogen-associated molecular patterns (PAMPs). The pattern recognition receptors belong to the family of Toll like receptors (TLRs).[15] Constantly, these TLRs have been found to be involved in activation of different signaling pathways, resulting in virus recognition, downstream transduction pathways, such as nuclear factor κB (NF-κB) and p38 MAPK (mitogen-activated protein kinases) which are crucial for antiviral response as shown in [Figure 1] .[16]
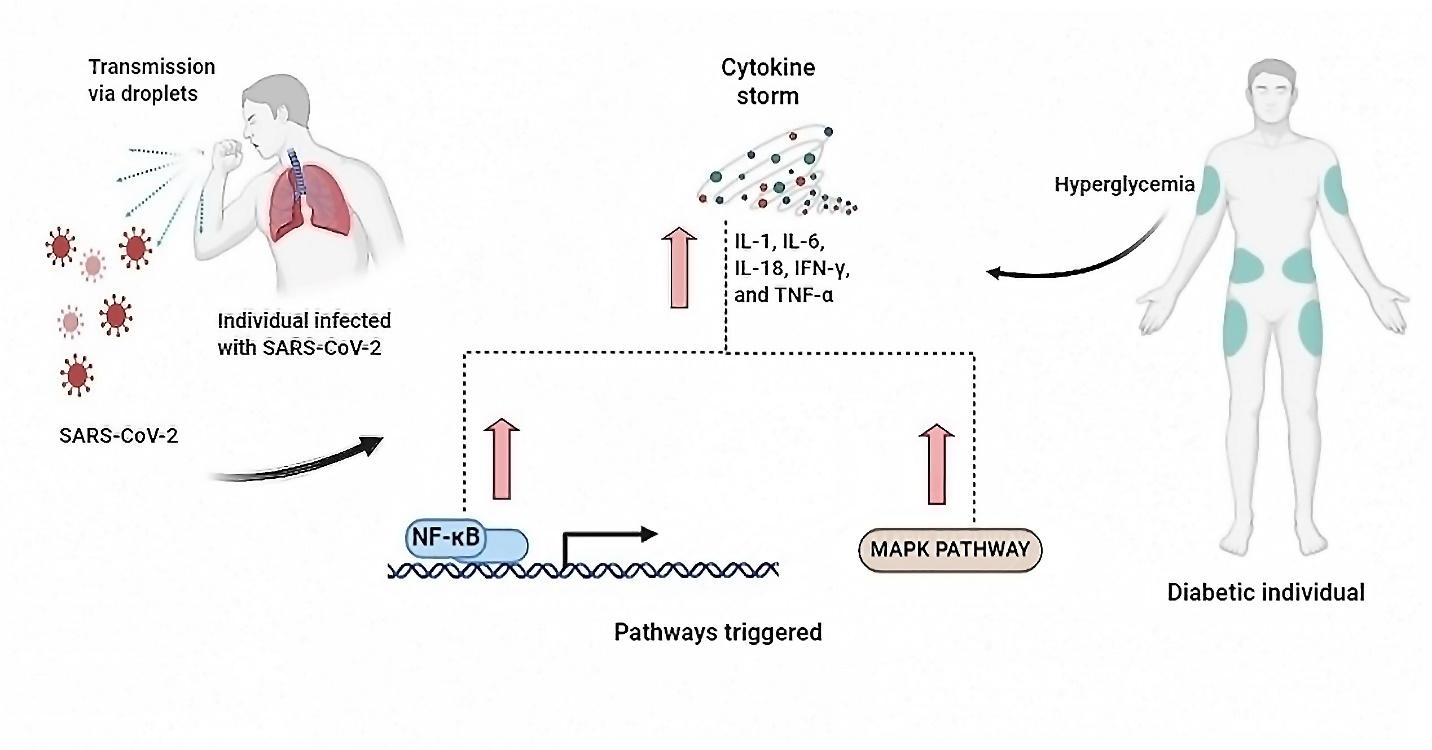
The NF-κB signaling pathway
In the classical NF-κB signaling pathway, the transcription factor NF-κB is one of the critical regulator of both adaptive and innate immunity.[17] Meanwhile, severe acute respiratory syndrome (SARS) is diagnosed by an uncontrolled inflammatory response. NF-κB is reported to be the major transcription factor triggered in ARDS.[18] NF-кB is a key player in mediating responses of inflammation and other cellular activities.[19] After NF-кB is triggered, it induces the release of cytokines, which later results in producing a positive autoregulatory loop and resulting in impairment of inflammatory response. In contrast, work done by Ma et al., 2020[20] evidenced that the NF-кB signaling pathway is activated through SARS-CoV-2 that could results in increase in the expression of the IκBα (inhibitor of nuclear factor kappa B) induced by SARS-CoV-2.
Many adhesion molecules, cytokines, chemokines, enzymes etc. which releases inhibitors of apoptosis, major histocompatibility complex class II antigens and secondary inflammatory mediators are triggered when NF-κB signaling pathway is triggered.[21] All the factors viz. IL-6, IL-1β, IL-8, intercellular adhesion molecule 1, vascular cell adhesion molecule 1, cyclooxygenase-2 and inducible NO-synthase (iNOS) help protection against pathogens as first line defense.[22] Moreover, any variations or errors in regulation of NF-κB, mainly up-regulation, can cause diabetic wound healing to be impaired. This is because NF-κB also controls genes accountable for cell proliferation.[23], [24] Taking above facts altogether, the above connecting data suggest that NF-κB inhibition could be an effective approach in prevention of this pathogenic disease.
The p38 MAPK signaling pathway
COVID-19 is a respiratory infection, and SARS-CoV-2 virus is responsible for it. Because of its inflammatory action, it affects the lungs, heart and results in becoming a major source of mortality and morbidity. p38 MAPK pathway upregulation triggers the release of pro-inflammatory cytokines such as IL-1β and IL-6. In contrast, IL-6 is known to cause acute lung injury and myocardial dysfunction.[25] Upon endocytosis binding of SARS-CoV-2 to host cell downregulates ACE2. After that, conversion of Angiotensin II into Angiotensin 1–7 take place with aid of ACE2. Binding of Angiotensin 1–7 to the Mas receptor after conversion takes place to counterbalance the vasoconstrictive and pro-inflammatory effects caused by Ang II.[26], [27] Therefore, decreased levels of ACE 2 lead to increased proinflammatory cytokines. Activation of additional pro-inflammatory cytokines such as iNOS and cyclooxygenase 2 (COX-2) is also done by the p38 MAPK pathway.[28], [29], [30] Different reports have also suggested that p38 MAPK is also found to be triggered in diabetic individuals in other tissues, including heart, nerve, kidney and vasculature[31], [32], [33], [34], [35], [36] Therefore, in diabetic patients inactivation of p38 MAPK regenerates cardiac function[37], [38] which is also the chief organ affected by SARS-CoV-2. This enables better survival chances of patients of cardiac myopathy or cardiac dysfunction.[25]
Dispersion of SARS-CoV2
Management of diabetes associated comorbidities during COVID-19 outbreak
Individuals having diabetes are susceptible to to psychological distress, anxiety and depression. Co-morbidities like these can lead in the direction of diabetes related complications which could affect the survival. Poor diet quality and inadequate social support are some of the main reasons behind these complications. Immunocompromised conditions worsen the situation with diabetics. The implementation of lockdown has a negative effect on the quality of life of people with diabetes. It has also decreased their physical activities and affected their glucose self-management. Subsequently, psychological aid and additional support are of utmost importance during the time of pandemic.[39] It is evaluated that 463 million individuals are affected by diabetes globally. It is noteworthy that if we enlist the nations with largest number of diabetic patients then we find again China to be leading countries. The rapid spread of COVID-19 thrived to become a global pandemic, affecting massively the health care sector.[40]
The International Diabetes Federation encourages individuals with diabetes to have the necessary supplies and equipment at their home to prevent them from wandering off when they become sick. The immunocompromised diabetic patients are always at a higher risk than the general non diabetic population. If diabetes patients suddenly feel sick, they might start to notice that their glycaemic level starts to deteriorate. This could be a warning sign that their condition is getting worse. In such cases, the patient should contact their family physician or other healthcare helpline to avoid visiting a hospital or clinic.[41]
|
Name of Vaccine |
Manufacturer |
Type |
Status |
Ref |
|
Covishield |
Serum Institute of India |
Recombinant, ChAdOx1 chimpanzee adenoviral vector, encoding SARS-CoV-2 spike protein. |
Finalized |
|
|
COVAXIN |
Bharat Biotech, India |
Whole-Virion Inactivated Vero Cell |
Finalized |
|
|
COMIRNATY |
Pfizer bioNTech |
Modified nucleoside mNRA |
Finalized |
|
|
Vaxzevria |
AstraZeneca |
Recombinant, ChAdOx1 chimpanzee adenoviral vector, encoding SARS-CoV-2 spike protein. |
Finalized |
|
|
mRNA-1273 |
Moderna Biotech |
Lipid nanoparticle (LNP) encapsulated mNRA-based vaccine |
Finalized |
|
|
SARS-CoV-2 Vaccine (Vero Cell) |
Sinopharm/ Beijing Institute of Biological Products Co., Ltd. (BIBP) |
Inactivated, produced in Vero cells |
Finalized |
|
|
CoronavacTM |
Sinovac Life Sciences Co., Ltd. |
Inactivated, produced in Vero cells |
Finalized |
|
|
Sputnik V |
The Gamaleya National Center |
based on Human Adenovirus Vector |
Ongoing |
Current vaccine treatment and its link to diabetics
Several vaccines are now presently being used for the treatment of COVID-19 lethal and many of them are under WHO EUL (Emergency Use Listing Procedure) evaluation for their potential use. The mass vaccination programme was first started in early December 2020. The number of vaccination doses administered is updated on WHO’s dashboard regularly.[50] In India, the Drug Controller General of India (DCGI) have approved COVAXIN™ and COVISHIELD™. The COVID-19 have a tendency to have a poor prognosis with diabetes patients. With several clinical data from different countries, vaccination in patients with diabetes is justified and highly encouraged.[51] The list of currently available COVID-19 vaccines and vaccines under assessment is given in [Table 1] .
Conclusion
The COVID-19 pandemic is biggest health challenge with 4,636,153 deaths reported so far globally on World Health Organization COVID-19 dashboard till 14 September 2021.[50] Persistence of this disease affecting humanity is difficult until development of targeted vaccine takes place. Therefore, this is the need of the hour to understand the necessity of saving humanity from an exposure to the virus, specifically when they are immunocompromised. Henceforth, precise care should be given to the diabetic patients during this pandemic to avoid addition of more difficulties and burden to the healthcare systems. Due to the nature of the co-morbidities involved in diabetes, it is important that the public is informed about the various vaccine options available to manage this condition.
Conflicts of Interest
Authors declare no conflict of interest.
Acknowledgement
The author SB acknowledges DST Inspire (IFA-12, LSBM-32) for the financial support and Department of Bioscience and Biotechnology, Banasthali Vidyapith, Rajasthan. The author SG acknowledges Department of Bioscience and Biotechnology, Banasthali Vidyapith, Rajasthan.
References
- Liu Z, Bing ZX, Zhi XZ. Epidemiology working group for NCIP epidemic response. Chinese Center for Disease Control and Prevention. Epidemiol Charact Outbr. 2019;41(2):145-51. [Google Scholar]
- . IDF diabetes atlas. . 2019. [Google Scholar]
- Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-13. [Google Scholar]
- Cuschieri S, Grech S. COVID-19 and diabetes: The why, the what and the how. J Diabetes Complications. 2020;34(9). [Google Scholar]
- Armstrong D, Boulton A, Bus S. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376(24):2367-75. [Google Scholar]
- Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Eng J Med. 2020;382(13):1199-1207. [Google Scholar]
- Adhikari S, Meng S, Wu Y, Mao Y, Ye R, Wang Q. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Povert. 2020;9(1):1-2. [Google Scholar]
- Jafar N, Edriss H, Nugent K. The effect of short-term hyperglycemia on the innate immune system. Am J Medic Sci. 2016;351(2):201-11. [Google Scholar]
- Singh A, Gupta R, Ghosh A, Misra A. Diabetes in COVID-19 Prevalence, pathophysiology, prognosis and practical considerations. Diabetes & Metabolic Syndrome. Clinic Res Rev. 2020;14(4):303-10. [Google Scholar]
- Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420-2. [Google Scholar]
- Shimizu M, Cron R, Behrens E. Clinical Features of Cytokine Storm Syndrome. Cytokine Storm Syndrome. 2019. [Google Scholar]
- Pal R, Bhansali A. COVID-19, diabetes mellitus and ACE2: the conundrum. Diabetes Res Clin Pract. 2020;162. [Google Scholar] [Crossref]
- South A, Tomlinson L, Edmonston D, Hiremath S, Sparks M. Controversies of renin-angiotensin system inhibition during the COVID-19 pandemic. Nat Rev Nephrol. 2020;16(6):305-7. [Google Scholar]
- Yang J, Lin S, Ji X, Guo L. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Act Diabetol. 2010;47(3):193-9. [Google Scholar]
- DeMarcken M, Dhaliwal K, Danielsen A, Gautron A, Dominguez-Villar M. TLR7 and TLR8 activate distinct pathways in monocytes during RNA virus infection. Sci Signal. 2019;12(605). [Google Scholar]
- Olejnik J, Hume AJ, Mühlberger E. Toll-like receptor 4 in acute viral infection: too much of a good thing. PLoS Pathog. 2018;14(12). [Google Scholar]
- Li Q, Verma I. NF-κB regulation in the immune system. Nat Rev Immunol. 2002;2(10):725-34. [Google Scholar]
- Fan J, Ye R, Malik A. Transcriptional mechanisms of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2001;281(5):1037-50. [Google Scholar]
- Santoro M, Rossi A, Amici C. NF-κB and virus infection: who controls whom. EMBO J. 2003;22(11):2552-60. [Google Scholar]
- Ma Q, Pan W, Li R, Liu B, Li C, Xie Y. Liu Shen capsule shows antiviral and anti-inflammatory abilities against novel coronavirus SARS-CoV-2 via suppression of NF-κB signaling pathway. Pharmacol Res. 2020;158. [Google Scholar]
- Ghosh S, May M, Kopp E. NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Ann Rev Immunol. 1998;16(1):225-60. [Google Scholar]
- Jobin C, Sartor R. The IκB/NF-κB system: a key determinant of mucosal inflammation and protection. Am J Physiol-Cel Physio. 2000;278(3):451-62. [Google Scholar]
- Na J, Lee K, Na W, Lee J, Lee M, TY. Histone H3K27 demethylase JMJD3 in cooperation with NF-κB regulates keratinocyte wound healing. J Invest Dermatol. 2016;136(4):847-58. [Google Scholar]
- Schreml S, Szeimies RM, Prantl L, Landthaler M, Babilas P. Wound healing in the 21st century. J Am Acad Dermatol. 2010;63(5):866-81. [Google Scholar]
- Zarubin T, Jiahuai H. Activation and signaling of the p38 MAP kinase pathway. Cel Res. 2005;15(1):11-8. [Google Scholar]
- Crowley S, Rudemiller N. Immunologic effects of the renin-angiotensin system. J Am Societ Nephrol. 2017;28(5):1350-61. [Google Scholar]
- Silva A, Silveira K, Ferreira A, Teixeira M. ACE2, angiotensin‐(1‐7) and M as receptor axis in inflammation and fibrosis. Br J Pharmacol. 2013;169(3):477-92. [Google Scholar]
- Badger A, Roshak A, Cook M, Newman-Tarr T, Swift B, Carlson K. Differential effects of SB 242235, a selective p38 mitogen-activated protein kinase inhibitor, on IL-1 treated bovine and human cartilage/chondrocyte cultures. Osteoarth Cartil. 2000;8(6):434-43. [Google Scholar]
- Guan Z, Buckman S, Pentland A, Templeton D, Morrison A. Induction of cyclooxygenase-2 by the activated MEKK1→ SEK1/MKK4→ p38 mitogen-activated protein kinase pathway. J Biol Chem. 1998;273(21):12901-8. [Google Scholar]
- Guan Z, Buckman S, Springer L, Morrison A. (eds) Eicosanoids and Other Bioactive Lipids in Cancer, Inflammation, and Radiation Injury. Adv Exp Med Biol. 1999;469:9-15. [Google Scholar]
- Igarashi M, Wakasaki H, Takahara N, Ishii H, Jiang Z, Yamauchi T. Glucose or diabetes activates p38 mitogen-activated protein kinase via different pathways. J Clinic Invest. 1999;103(2):185-95. [Google Scholar]
- Adhikary L, Chow F, Nikolic-Paterson D, Stambe C, Dowling J, Atkins R. Abnormal p38 mitogen-activated protein kinase signalling in human and experimental diabetic nephropathy. Diabetologia. 2004;47(7):1210-22. [Google Scholar]
- Chen H, Brahmbhatt S, Gupta A, Sharma A. Duration of streptozotocin-induced diabetes differentially affects p38-mitogen-activated protein kinase (MAPK) phosphorylation in renal and vascular dysfunction. Cardiovasc Diabetol. 2005;4(1):1-3. [Google Scholar]
- Komers R, Lindsley J, Oyama T, Cohen D, Anderson S. Renal p38 MAP kinase activity in experimental diabetes. Lab Invest. 2007;87(6):548-58. [Google Scholar]
- Igarashi M, Hirata A, Yamaguchi H, Sugae N, Kadomoto-Antsuki Y, Nozaki H. Characterization of activation of MAP kinase superfamily in vasculature from diabetic rats. J Atheroscler Thromb. 2007;14(5):235-44. [Google Scholar]
- Agthong S, Tomlinson D. Inhibition of p38 MAP kinase corrects biochemical and neurological deficits in experimental diabetic neuropathy. Ann New York Acad Sci. 2002;973(1):359-62. [Google Scholar]
- Westermann D, Rutschow S, Linthout SV, Linderer A, Bücker-Gärtner C, Sobirey M. Inhibition of p38 mitogen-activated protein kinase attenuates left ventricular dysfunction by mediating pro-inflammatory cardiac cytokine levels in a mouse model of diabetes mellitus. Diabetologia. 2006;49(10):2507-13. [Google Scholar]
- Thandavarayan R, Watanabe K, Ma M, Gurusamy N, Veeraveedu P, Konishi T. Dominant-negative p38α mitogen-activated protein kinase prevents cardiac apoptosis and remodeling after streptozotocin-induced diabetes mellitus. Am J Physiol Heart Circ Physiol. 2009;297(3):911-9. [Google Scholar]
- Mukhtar S, Mukhtar S. Letter to the Editor: Mental Health and Psychological Distress in People with Diabetes during COVID-19. Metabolism. 2020;108. [Google Scholar]
- Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y. Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis. Ann med. 2017;49(2):106-16. [Google Scholar]
- . International Diabetes Federation (IDF). COVID-19 outbreak: guidance for people with diabetes. . 2020. [Google Scholar]
- Lampasona V, Secchi M, Scavini M, Bazzigaluppi E, Brigatti C, Marzinotto I. Antibody response to multiple antigens of SARS-CoV-2 in patients with diabetes: an observational cohort study. Diabetol. 2020;63(12):2548-58. [Google Scholar]
- . U.S. Food and Drug Administration. FDA Approves First COVID-19 Vaccine. . . [Google Scholar]
- Dispinseri S, Lampasona V, Secchi M, AC, Bazzigaluppi E, DN. Robust neutralizing antibodies to SARS-CoV-2 develop and persist in subjects with diabetes and COVID-19 pneumonia. J Clin Endocrinol Metab. 2021;106(5):1472-81. [Google Scholar]
- . Sinovac announces phase III results of its COVID-19 vaccine. . . [Google Scholar]
- . Centers for Disease Control and Prevention. Moderna COVID-19 Vaccine Overview and Safety. . . [Google Scholar]
- . World Health Organization (WHO). Sinopharm [Vero Cell]- Inactivated, COVID-19 vaccine. . . [Google Scholar]
- . World Health Organization (WHO). The Sinovac-CoronaVac COVID-19 vaccine: What you need to know. . . [Google Scholar]
- Nogrady B. Mounting evidence suggests Sputnik COVID vaccine is safe and effective. Nature. 2021;595(7867):339-40. [Google Scholar]
- . World Health Organization (WHO). Coronavirus disease 2019 Dashboard. . . [Google Scholar]
- Pal R, Bhadada S, Misra A. COVID-19 vaccination in patients with diabetes mellitus: Current concepts, uncertainties and challenges. Diabetes Metab Syndr. 2021;15(2):505-8. [Google Scholar]
- Abstract
- Introduction
- Etiology
- Diabetics and Cytokine Storm
- COVID-19 Triggered Pathways and their Possible Links with Diabetes
- Dispersion of SARS-CoV2
- Management of diabetes associated comorbidities during COVID-19 outbreak
- Current vaccine treatment and its link to diabetics
- Conclusion
- Conflicts of Interest
- Acknowledgement
- References
How to Cite This Article
Vancouver
Gaur S, Bajpai S, Gaur S, Singhal S, Mishra R. Coronavirus disease 2019 in diabetes: A pathophysiological linkage [Internet]. Int J Clin Biochem Res. 2022 [cited 2025 Sep 23];9(2):92-97. Available from: https://doi.org/10.18231/j.ijcbr.2022.019
APA
Gaur, S., Bajpai, S., Gaur, S., Singhal, S., Mishra, R. (2022). Coronavirus disease 2019 in diabetes: A pathophysiological linkage. Int J Clin Biochem Res, 9(2), 92-97. https://doi.org/10.18231/j.ijcbr.2022.019
MLA
Gaur, Shreshtha, Bajpai, Surabhi, Gaur, Sonal, Singhal, Sonu, Mishra, Rakesh. "Coronavirus disease 2019 in diabetes: A pathophysiological linkage." Int J Clin Biochem Res, vol. 9, no. 2, 2022, pp. 92-97. https://doi.org/10.18231/j.ijcbr.2022.019
Chicago
Gaur, S., Bajpai, S., Gaur, S., Singhal, S., Mishra, R.. "Coronavirus disease 2019 in diabetes: A pathophysiological linkage." Int J Clin Biochem Res 9, no. 2 (2022): 92-97. https://doi.org/10.18231/j.ijcbr.2022.019
