- Visibility 606 Views
- Downloads 131 Downloads
- Permissions
- DOI 10.18231/j.ijcbr.2024.026
-
CrossMark
- Citation
sFlt-1/PLGF ratio: A promising marker for early detection of preeclampsia in the second and third trimester
- Author Details:
-
Mandeep Kaur *
-
Sahiba Kukreja
-
Siddhant Arora
-
Sukhjeet Kaur
-
Manmeet Kaur Gill
Abstract
Introduction: Preeclampsia (PE), characterized by endothelial dysfunction, remains a significant concern in obstetrics due to its association with maternal and fetal morbidity and mortality. One significant contributor to the clinical manifestations of PE is the imbalance in the placental release of various angiogenesis regulatory factors into the maternal circulation. Low levels of the pro-angiogenic biomarker Placental Growth Factor (PLGF) and high levels of antiangiogenic biomarker sFlt-1 (soluble Fms like tyrosine kinase -1) levels are detectable several weeks before the clinical presentation of PE, making them a promising marker for early diagnosis.
Aim: This study investigates the utility of the sFlt-1/PLGF ratio in predicting and diagnosing preeclampsia during the second and third trimesters of pregnancy.
Materials and Methods: A prospective cohort study was conducted with 150 study participants comprising normotensive controls and preeclamptic cases, diagnosed based on blood pressure and proteinuria criteria. Serum samples collected in the second trimester (24-28 weeks) and third trimester (>28 weeks) were analyzed for PLGF and sFlt-1 levels using ELISA kit method. The sFlt-1/PLGF ratio was calculated and evaluated for its diagnostic accuracy through ROC curve analysis.
Results: Significantly lower PLGF levels and higher sFlt-1 levels in preeclamptic pregnancies compared to normotensive pregnancies were seen in both trimesters (p < 0.001). The sFlt-1/PLGF ratio was markedly elevated in preeclampsia, showing strong predictive characteristics with an AUC of 0.929 (sensitivity 90%, specificity 90%) in the second trimester and an AUC of 0.986 (sensitivity 90%, specificity 96.7%) in the third trimester. These findings highlight the potential of the sFlt-1/PLGF ratio as a biomarker for early detection and risk stratification in pregnancies complicated by preeclampsia.
Introduction
Hypertension in pregnancy is classified by the American College of Obstetricians and Gynecologists (ACOG) into four categories: pre-eclampsia-eclampsia, chronic hypertension, chronic hypertension with superimposed preeclampsia, and gestational hypertension. Hypertensive disorders affect 7.8% of pregnancies, with specific prevalence for preeclampsia (5.6%), gestational hypertension (1.5%), chronic hypertension (0.15%), and eclampsia (0.60%). Preeclampsia is a major cause of maternal mortality, responsible for around 60,000 maternal and 500,000 perinatal deaths annually.[1] Early delivery of the placenta and fetus is the most effective treatment. If untreated, the condition can progress to multi-organ dysfunction. WHO identifies it as the second leading direct cause of maternal mortality, following hemorrhage. Preexisting diagnostic markers like hypertension and proteinuria are of limited value for diagnosis in cases of preexisting hypertension or proteinuria, as in chronic renal diseases. Recent work identified few novel angiogenic factors that are linked to the disease's pathogenesis.[2]
Preeclampsia is primarily an endothelial disease marked by endothelial dysfunction. Endothelial cell layer becomes leaky, lose their typical flat morphology and function. Angiogenic biomarkers, particularly the sFlt-1/PLGF ratio, is considered useful for early prediction and diagnosis of preeclampsia and other related conditions. Various studies have shown that changes in sFlt-1 and PLGF markers can be detected as early as the second trimester. The sFlt-1/PLGF ratio aids in predicting and diagnosing the condition, with elevated levels observed in the second trimester of pregnancies complicated by preeclampsia.[3] Elevated sFlt-1/PLGF ratios reflect inefficient placentation and placental hypoxia. This ratio may also help distinguish preeclampsia from other hypertensive disorders and in patients with chronic hypertension suspected to develop superimposed preeclampsia. These early marker alterations can reduce morbidity and mortality through early risk stratification. [4] Adopting a standardized screening approach using the sFlt-1/PLGF ratio alongside uterine Doppler velocimetry could enhance early detection of pre-eclampsia and other conditions related to the placenta.[5] Numerous studies have also shown that sFlt-1 and PLGF levels can serve as effective prognostic tests, correlating with pregnancy duration in early-onset preeclampsia. [6], [7] The PROGNOSIS study validated cut-off values for short-term preeclampsia prediction, recommending that an sFlt-1/PLGF ratio below 38 rules out preeclampsia onset within one week in suspected cases.[8]
Although understanding of these processes has improved, managing preeclampsia has not advanced correspondingly. Current diagnostic criteria are still based on nonspecific clinical, ultrasound, and laboratory findings rather than their pathogenic origins, with little change over decades. This study aims to fill gaps in knowledge by investigating the association of sFlt-1/PLGF ratio with the second and third trimesters of pregnancy complicated by Preeclampsia.[9] In India, where preeclampsia prevalence is high, the use of sFlt-1/PLGF ratio could aid in patient management but further research is needed to validate findings.
Materials and Methods
Study Design: This prospective cohort study was conducted in the Department of Biochemistry, in collaboration with the Department of Obstetrics and Gynecology at a tertiary care hospital. The study spanned from December 2018 to March 2022, following approval from the Institutional Research and Ethical Committee. (IEC Patho 899/18 dated 23.10.2018)
Inclusion and exclusion criteria
A total of 150 participants in their second trimester of pregnancy (24-28 weeks) in the age group of 20-40 years were included in the study and were selected through consecutive sampling from the Obstetrics and Gynecology department. Women with chronic medical conditions such as renal diseases, cardiac diseases, diabetes mellitus, previous hypertension were excluded from the study group. The sample size was calculated using Epi Info software version 7.2.2.6, taking reference from previous article (12) and was found to be 110 at 95% confidence interval and a 5% alpha error. Accounting for potential dropouts, the sample size was set at 150. Informed written consent was obtained from all participants before enrollment. Participants were divided into two groups based on blood pressure and proteinuria measurements:-
Group 1 (Normotensive controls): Pregnant women with normal blood pressure and either negative proteinuria or urine protein levels of +1.
Group 2 (Preeclamptic cases): Pregnant women with newly diagnosed hypertension (systolic BP ≥ 140 mmHg and/or diastolic BP ≥ 90 mmHg on two occasions at least 4 hours apart) and proteinuria (urine protein levels of +2 or more after 20 weeks of gestation). Blood pressure was measured using a calibrated sphygmomanometer with participants in the sitting position after 5-minute rest. Urinary protein estimation was conducted using a semi-quantitative dipstick assay. Participants with a history of hypertension, renal diseases, cardiac diseases, diabetes mellitus, smoking, or drinking were excluded. Data on demographic, general, and obstetric characteristics were collected using a validated form.
Follow-Up: All participants from both the groups were followed up in the third trimester (beyond 28 weeks).
Methodology
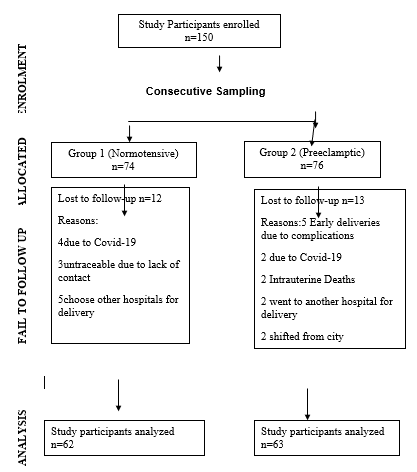
Four milliliters of venous blood was drawn from participants under aseptic conditions and kept at room temperature for 30 minutes. The samples were centrifuged for 17 minutes at 3500 rpm to separate serum, which was then stored at -20°C. The first blood sample was collected during the second trimester (24-28 weeks), and the second sample was taken in the third trimester (beyond 28 weeks) for PLGF and sFlt-1 estimation ([Figure 1]). Serum PLGF and sFlt-1 levels were measured using commercially available ELISA kits from Qayee-Biochemicals. Ratio of sFlt-1/ PLGF was calculated.
Statistical analysis
Continuous demographic data were analyzed as mean ± standard deviation (SD), while categorical data was presented as percentages. Differences between study groups were compared using the student’s t-test. The chi-square test was used for qualitative categorical data. PLGF and sFlt-1 data were represented as median and interquartile range. The Mann-Whitney U test and the Wilcoxon signed-rank test were used to analyze PLGF, sFlt-1 and sFlt-1/ PLGF data. ROC curve analysis was performed on second and third trimester data of ratio of sFlt-1/ PLGF to calculate the area under the curve (AUC), optimal cut-off values, sensitivity, specificity, lower and upper bounds for the 95% confidence interval, negative predictive values (NPV), and positive predictive values (PPV). A p-value < 0.001 was considered highly significant, and a p-value < 0.05 was considered significant for all tests.
Results
As shown in [Figure 1] which represents the flow chart of study design, 74 participants were enrolled in group 1 and 76 in group 2. Twelve participants from group 1 and thirteen from group 2 were lost to follow-up. Demographic and obstetric characteristics were analyzed, revealing significant differences in mean values between the preeclamptic and normotensive groups ([Table 1]).
Summarizes the serum PLGF concentrations in median and interquartile range(IQR) during the second and third trimesters for both groups. PLGF concentrations were consistently lower in the preeclamptic group compared to the normotensive group. In the second trimester, the median PLGF concentration was 12.36 in the preeclamptic group, significantly lower than the median of 16.25 in the normotensive group(p<0.001) In the third trimester, the median PLGF concentration was 13.64 in the normotensive group, whereas it was 10.05 in the preeclamptic group, showing a statistically significant difference (p < 0.001).
Levels of the sFlt-1 were observed to be elevated in the group 2 with preeclampsia compared to the normotensive group during both trimesters. ([Table 3]) In the second trimester, the median value was 66.15 in the normotensive group, significantly lower than the median of 315.05 in the preeclamptic group (p < 0.001). Similarly, in the third trimester, the normotensive group had a median value of 77.10 compared to 338.68 in the preeclamptic group, which was also significantly different (p < 0.001).
As shown in [Table 4] on calculating ratio of sFlt-1/ PLGF, the median in the normotensive group was 4.07, which is lower than the median value 25.49 in the preeclamptic group, the difference being statistically highly significant with p < 0.001. Third trimester median value was 5.65 ng/ml in normotensive group, as compared to the preeclamptic group with median value of 33.7and the difference is found to be statistically highly significant p < 0.001. As shown in [Figure 2] data is graphically represented in form of the Box Plot Analysis.
[Figure 3] shows the predictive characteristics of biomarkers with reference to AUC obtained during ROC curve analysis of second and third trimester data. The predictive characteristics of sFlt-1/PLGF showed sensitivity 90%, specificity 90%, PPV 90%, NPV 89.5% with AUC 0.929 at 15.51 software defined cut off point in second trimester. The predictive characteristics of sFlt-1/PLGF showed diagnostic accuracy 93.3%, sensitivity 90%, specificity 96.7%, PPV 96.4%, NPV 90.6% with AUC 0.986 at cutoff point 25.79 in third trimester. ([Table 5])



|
S.NO |
Charateristics |
Normotensive group |
Preeclamptic group |
p value |
|
1. |
Maternal Age (years) |
26.10 ± 3.07 |
30.75 ± 5.52 |
<0.001 |
|
2. |
Maternal weight (Kg) |
58.8 ±6.02 |
69.89 ±8.02 |
<0.001 |
|
3. |
Maternal height (ft | in) |
5.36 ± 0.19 |
5.22±0.11 |
<0.001 |
|
4. |
Systolic Blood Pressure(mmHg) |
108.05 ± 11.05 |
154.19 ± 9.91 |
<0.001 |
|
5. |
Diastolic Blood Pressure(mmHg) |
71.63 ± 8.07 |
101.83 ± 6.17 |
<0.001 |
|
6. |
Proteinuria |
3 (4.8%) |
56 (90.3%) |
<0.001 |
|
7. |
Gravidity Primigravidae Multigravidae |
14 (22.5%) 48 (77.41%) |
26 (41.2%) 37 (58.3%) |
0.002 |
|
8. |
Previous history of PIH (pregnancy induced hypertension) present |
1(1.58%) |
35(55.5%) |
<0.001 |
|
9. |
Family history of hypertension present |
7(11.29%) |
16 (25.3%) |
0.015 |
|
Group S |
PLGF (ng/mL) |
Second trimester levels |
Third trimester levels |
p value |
|
Normotensive group |
Median |
16.25 |
13.64 |
p<0.001 |
|
IQR |
15.01 -17.52 |
12.02 -15.98 |
||
|
Preeclamptic group |
Median |
12.36 |
10.05 |
p<0.001 |
|
IQR |
10.29-13.94 |
8.67-11.05 |
||
|
p value |
p<0.001 |
p<0.001 |
|
|
|
sFlt-1(ng/mL) |
Second trimester levels |
Third trimester levels |
p value |
|
Normotensive group |
Median |
66.15 |
77.105 |
<0.001 |
|
IQR |
54.10 -78.97 |
60.97 -89.91 |
||
|
Preeclamptic group |
Median |
315.05 |
338.68 |
<0.001 |
|
IQR |
287.27 -332.91 |
347.25 -412.09 |
||
|
p value |
<0.001 |
<0.001 |
|
|
Groups |
sFlt-1/PLGF (ng/mL) |
Second trimester levels |
Third trimester levels |
p value |
|
Normotensive group |
Median |
4.07 |
5.65 |
p<0.001 |
|
IQR |
3.48 -4.76 |
4.89 -6.22 |
||
|
Preeclamptic group |
Median |
25.49 |
33.7 |
p<0.001 |
|
IQR |
20.37 – 27.82 |
29.64 -40.79 |
||
|
|
p<0.001 |
p<0.001 |
|
|
sFlt-1/PLGF |
Sensitivity % |
Specificity % |
PPV% |
NPV% |
|
2nd trimester |
90 |
90 |
90 |
90 |
|
3rd trimester |
90 |
96.7 |
96.4 |
90.6 |
|
Group 2 No. of participants (%) |
Ratio cut off<38 |
Ratio cut off >38 |
|
2nd trimester n (%) |
57 (90.4%) |
6 (9.4%) |
|
3rd trimester n (%) |
37(58.7%) |
26 (41.3%) |
Discussion
Preeclampsia (PE) is a serious complication of pregnancy and a significant contributor to maternal and neonatal health risks globally. [10] Already established diagnostic markers like hypertension and proteinuria lack precision in predicting disease progression and outcomes. [11], [12] Accurate and timely diagnosis remains challenging.[13] Recent research emphasizes the role of placental angiogenic factors, specifically Placental Growth Factor (PLGF), sFLT-1 and ratio of sFlt-1/ PLGF as crucial biomarkers for PE.[14]
The findings of the current study indicated significant relationships between preeclampsia and family history of hypertension, previous PIH, advanced maternal age, nulliparity, being overweight, proteinuria, and levels of blood pressure as shown in [Table 1], which is similar to the previous studies.[13] Various recent studies also indicate that advanced maternal age increase the risk of preeclampsia by two fold. In the current study Preeclampsia was observed in patients of advanced maternal age, which also aligns with findings in other studies. The published literature also indicates a higher prevalence of preeclampsia in older age groups compared to younger ones.Duckitt and Harrington (2005) found that extremes in childbearing age (very young or older) are associated with an increased risk of preeclampsia/eclampsia.[15] Lamminpaa et al. (2012) found that women aged 40 or older have nearly double the risk of preeclampsia compared to women under 35, even after accounting for baseline differences, emphasizing that advanced maternal age remains a significant risk factor independent of parity.[16] Nulliparity and family history increase the risk by threefold. Elevated BMI increases the preeclampsia risk by many folds, even within the normal range.[15], [16]
In the present study, during second and third trimester of pregnancy, levels of PLGF were found to be significantly lower and soluble Fms-like tyrosine kinase-1 (sFlt-1) levels were significantly elevated in women with preeclampsia, in consistence with previously reported findings. As depicted in [Table 2] when PLGF levels between two groups were compared, PLGF levels were significantly lower in second and third trimester of preeclamptic group (group2) when compared with normotensive group (group1) with median 16.25 versus 12.36 (p value<0.001) in second and 13.64 versus 10.05 (p value<0.001) in third trimesters respectively. In the current study as shown in table 3, on comparison of sFlt-1 levels between two groups, levels were significantly higher in preeclampsia group (group2) both in second and third trimesters when compared with normotensive group (group1) with median 315.05 versus 66.15 (p value<0.001) and 338.68 versus 77.10 (p value < 0.001) respectively. Elevated levels of soluble Fms-like tyrosine kinase-1 bind PLGF and vascular endothelial growth factor, disrupting angiogenic balance and leading to endothelial dysfunction with resultant clinical manifestations of preeclampsia. Incorporating the estimation of these angiogenic markers could enhance early detection, improve management strategies, and potentially reduce severe maternal complications associated with PE.[17], [18] Results of the study were consistent with the previous prospective cohort studies done by Antonio De Vivo et al.[19] and Radulescu et al.[11] showing PLGF levels to be lower and sFlt-1 to be higher in preeclamptic group in both trimesters.
As shown in [Table 4] in the present study sFlt-1/ PLGF ratio levels were significantly higher in preeclamptic group in both the trimesters with median 25.49 (IQR 20.37 – 27.82) versus 4.07 (IQR 3.48 -4.76) (p value <0.001) in second and 33.7 (IQR 29.64 -40.79) versus 5.65 (IQR 4.89 -6.22) (p value <0.001) in third trimesters respectively. Results in the current study are in accordance with previously published studies. [19] De Vivo et al. (2008) conducted a cohort study showing significant differences in second and third trimester median sFlt-1/PLGF levels between preeclamptic and control groups (106.7 vs 12.2 in the second trimester, 411.2 vs 19.2 in the third trimester; p < 0.001.[20] They identified sFlt-1/PLGF ratio in maternal serum as a highly accurate predictor of preeclampsia during both trimesters. Gurnadi et al. (2015) similarly found significantly different sFlt-1/PLGF ratios in severe preeclampsia versus normal pregnancies (p < 0.001), indicating a substantial diagnostic difference with almost 15 times greater difference in both the groups.[21] With reference to AUC obtained during ROC curve analysis of sFlt-1/PLGF the current study shows that sFlt-1/PLGF can be used as early predictive marker of preeclampsia with diagnostic accuracy 90%, sensitivity 90%, specificity 90%, PPV 90%, NPV 89.5% with AUC 0.929 at 15.51 cut off point in second trimester and with diagnostic accuracy 93.3%, sensitivity 90%, specificity 96.7%, PPV 96.4%, NPV 90.6% with AUC 0.986 at cutoff point 25.79 in third trimester.
Zeisler et al. (2016) have shown that the levels of sFlt-1/PLGF are dynamic, thus in women who had a sFlt-1/PLGF ratio of ≤ 38 at Visit 1 or > 38 to < 85 at initial visits, an increase in sFlt-1/PLGF ratio on retesting in the following 2 or 3 weeks might indicate pre-eclampsia in the near future or a higher risk of developing it.[22] Their observations showed that among women with levels ≤ 38 at Visit 1, nine patients developed pre-eclampsia at Visit 3 and three patients developed pre-eclampsia at Visit 4. These patients following retesting after 2–3 weeks were ruled in with a sFlt-1/PLGF ratio > 38. Top of FormBottom of FormZeisler et al. (2019) reported that a ratio >38 identified individuals at higher risk of preterm delivery (<37 weeks), whereas a low ratio of ≤38 effectively ruled out progression to pre-eclampsia within 2 to 3 weeks, showing a high negative predictive value (97.9% and 95.7%, respectively) in women suspected of developing pre-eclampsia before 37 weeks of gestation. Based on these previous publications and literature, in the current study as shown in table 6 on using a sFlt-1/PLGF cut-off value of 38 in preeclamptic patients during the second trimester, 90.4% of patients (57 out of 63) had a ratio < 38, while 9.6% (6 out of 63) had a ratio > 38. On retesting in the third trimester, 58.7% of patients (37 out of 63) had a ratio < 38, whereas 41.3% (26 out of 63) had a ratio > 38.Verlohren et al. (2012) observed that monitoring the sFlt-1/PLGF ratio in already-diagnosed patients could provide valuable prognostic information, correlating with the duration of pregnancy.[23] The elevated sFlt-1/PLGF ratio closely mirrors the underlying issues of inadequate placental function, hypoxia, and ischemia, proving more predictive of preeclampsia compared to measuring sFlt-1 or PLGF alone.[2] Studies suggest that monitoring this ratio between prenatal visits provides precise information, aiding clinicians in decision-making and offering prognostic insights for managing diagnosed patients. Diagnosing preeclampsia in women before severe complications from placental dysfunction also boosts clinicians' certainty when making decisions about terminating the pregnancy. Baltajian et al. (2016) conducted a study indicating a significant association between subsequent changes in the sFlt-1/PLGF ratio and the onset of pre-eclampsia. Their findings highlighted the importance of reassessing the sFlt-1/PLGF ratio in patients suspected of having pre-eclampsia. Furthermore, the sFlt-1/PLGF ratio serves as a predictive tool for adverse maternal and fetal outcomes, independent of the current standard diagnostic criteria.[24] Further research validating these findings, particularly in high-prevalence regions like India, is crucial for integrating this biomarker into clinical practice to improve maternal and fetal outcomes through early intervention strategies.
Conclusion
The study indicates that the ratio of disease-specific biomarkers sFlt-1/PLGF may serve as an effective predictor and a valuable biochemical marker for screening and diagnosing preeclampsia. It might be used as sensitive laboratory test for screening preeclampsia. Utilizing thesFlt-1/PLGF ratio may aid in the early detection of preeclampsia, potentially enhancing health outcomes by minimizing unnecessary hospitalizations for suspected cases. Additionally, it could impact management decisions, leading to improved monitoring and treatment protocols.
Source of Funding
None.
Conflict of Interest
None.
References
- Khan B, Yar R, Khakwani A, Karim S, Ali H. Preeclampsia incidence and its maternal and neonatal outcomes with associated risk factors. Cureus. 2022;14(11). [Google Scholar]
- Powe C, Levine R, Karumanchi S. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. . Circulation. 2011;123(24):2856-69. [Google Scholar]
- Rajan R. Role of sFlt-1 and PlGF ratio in the diagnosis, prediction and prognosis of pre-eclampsia: a review of literature with highlights from real World Indian experience. Pan Asian J Obs Gyn. 2018;1(1):24-30. [Google Scholar]
- Verlohren S, Herraiz I, Lapaire O, Schlembach D, Zeisler H, Calda P. New gestational phase-specific cutoff values for the use of the soluble fms-like tyrosine kinase-1/placental growth factor ratio as a diagnostic test for preeclampsia. Hypertension. 2014;63(2):346-52. [Google Scholar]
- Velegrakis A, Kouvidi E, Fragkiadaki P, Sifakis S. Predictive value of the sFlt‑1/PlGF ratio in women with suspected preeclampsia: An update. Int J Mol Med. 2023;52(4):1-8. [Google Scholar]
- Chaiworapongsa T, Romero R, Korzeniewski S, Cortez J, Pappas A, Tarca A. Plasma concentrations of angiogenic/anti-angiogenic factors have prognostic value in women presenting with suspected preeclampsia to the obstetrical triage area: a prospective study. . J Mat-fetal Neonatal Med. 2014;27(2):132-76. [Google Scholar]
- Chappell L, Duckworth S, Seed P, Griffin M, Myers J, Mackillop L. Diagnostic accuracy of placental growth factor in women with suspected preeclampsia: a prospective multicenter study. Circulation. 2013;128(19):2121-52. [Google Scholar]
- Zeisler H, Llurba E, Chantraine F, Vatish M, Staff A, Sennström M. Soluble fms-like tyrosine kinase-1 to placental growth factor ratio: ruling out pre-eclampsia for up to 4 weeks and value of retesting. . Ultrasound Obstet Gynecol. 2019;53(3):367-75. [Google Scholar]
- Herraiz I, Simón E, Gómez-Arriaga P, Moratalla J, Burguillo A, Jimenez L. Angiogenesis-related biomarkers (sFlt-1/PLGF) in the prediction and diagnosis of placental dysfunction: an approach for clinical integration. . Int J Mol Sci. 2015;16(8):19009-35. [Google Scholar]
- Nikuei P, Rajaei M, Roozbeh N, Mohseni F, Poordarvishi F, Azad M. Diagnostic accuracy of sFlt1/PlGF ratio as a marker for preeclampsia. BMC Pregnancy Childbirth. 2020;20(1):1-6. [Google Scholar]
- Radulescu C, Bacarea A, Hut A, Gabor R, Dobreanu M. Placental growth factor, soluble fms-like tyrosine kinase 1, soluble endoglin, IL-6, and IL-16 as biomarkers in preeclampsia. Med Inf. 2016. [Google Scholar]
- WZ. American College of Obstetricians and Gynecologists. Hypertension in pregnancy.. Int J Gynecol Obstet. 1996;53(2):175-83. [Google Scholar]
- Duhig K, Webster L, Sharp A, Gill C, Seed P, Shennan A. Diagnostic accuracy of repeat placental growth factor measurements in women with suspected preeclampsia: A case series study. Acta Obstetricia Et Gynecologica Scan. 2020;99(8):994-1024. [Google Scholar]
- Liao L, Zhao X, Zhou M, Deng Y, Li Y, Peng C. sFlt-1: A double regulator in angiogenesis-related diseases. Curr Pharm Des. 2021;27(40):4160-70. [Google Scholar]
- Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ. 2005;330(7491). [Google Scholar]
- Lamminp R, Julkunen K, Gissler M, Heinonen S. Preeclampsia complicated by advanced maternal age: a registry-based study on primiparous women in Finland 1997-2008. . BMC Preg Childbirth. 2012;12:1-5. [Google Scholar]
- Kaur M, Pahwa S, Arora R, Chhabra N, Kaur J, Kukreja S. Antiangiogenic Biomarker Soluble Fms-like Tyrosine Kinase-1 in Pregnancy Complicated with Preeclampsia: A Cohort Study. J Clin Diagn Res. 2021;15(12):11-5. [Google Scholar]
- Kaur M, Kukreja S, Chhabra N, Batish I, Pahwa S. Angiogenic biomarker placental growth factor (PLGF) in the prediction and diagnosis of placental dysfunction in pre-eclampsia: a cohort study. J Clin Diagn. 2023;17(6):1-5. [Google Scholar]
- Pant V, Yadav B, Sharma J. A cross-sectional study to assess the sFlt-1: PLGF ratio in pregnant women with and without preeclampsia. BMC Pregnancy Childbirth. 2019;19(1):1-8. [Google Scholar]
- Vivo D, Baviera A, Giordano G, Todarello D, Corrado G. PLGF and sFlt-1 as markers for predicting pre-eclampsia.. Acta Obstetricia Gynecologica Scandinavica. 2008;47(7):837-79. [Google Scholar]
- Gurnadi J, Mose J, Handono B, Satari M, Anwar A, Fauziah P. Difference of concentration of placental soluble fms-like tyrosine kinase-1 (sFlt-1), placental growth factor (PlGF), and sFlt-1/PlGF ratio in severe preeclampsia and normal pregnancy. . BMC Res Notes. 2015;38(2):1-5. [Google Scholar]
- Zeisler H, Llurba E, Chantraine F, Vatish M, Staff A, Sennström M. Predictive value of the sFlt-1: PlGF ratio in women with suspected preeclampsia. New Eng J Med. 2016;374(1):13-22. [Google Scholar]
- Verlohren S, Herraiz I, Lapaire O, Schlembach D, Moertl M, Zeisler H. The sFlt-1/PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstetrics Gynecol. 2012;206(1):58-9. [Google Scholar]
- Baltajian K, Bajracharya S, Salahuddin S, Berg A, Geahchan C, Wenger J. Sequential plasma angiogenic factors levels in women with suspected preeclampsia. . Am J Obstet Gynecol. 2016;215(1):89-90. [Google Scholar]
How to Cite This Article
Vancouver
Kaur M, Kukreja S, Arora S, Kaur S, Gill MK. sFlt-1/PLGF ratio: A promising marker for early detection of preeclampsia in the second and third trimester [Internet]. Int J Clin Biochem Res. 2024 [cited 2025 Oct 19];11(3):171-177. Available from: https://doi.org/10.18231/j.ijcbr.2024.026
APA
Kaur, M., Kukreja, S., Arora, S., Kaur, S., Gill, M. K. (2024). sFlt-1/PLGF ratio: A promising marker for early detection of preeclampsia in the second and third trimester. Int J Clin Biochem Res, 11(3), 171-177. https://doi.org/10.18231/j.ijcbr.2024.026
MLA
Kaur, Mandeep, Kukreja, Sahiba, Arora, Siddhant, Kaur, Sukhjeet, Gill, Manmeet Kaur. "sFlt-1/PLGF ratio: A promising marker for early detection of preeclampsia in the second and third trimester." Int J Clin Biochem Res, vol. 11, no. 3, 2024, pp. 171-177. https://doi.org/10.18231/j.ijcbr.2024.026
Chicago
Kaur, M., Kukreja, S., Arora, S., Kaur, S., Gill, M. K.. "sFlt-1/PLGF ratio: A promising marker for early detection of preeclampsia in the second and third trimester." Int J Clin Biochem Res 11, no. 3 (2024): 171-177. https://doi.org/10.18231/j.ijcbr.2024.026
